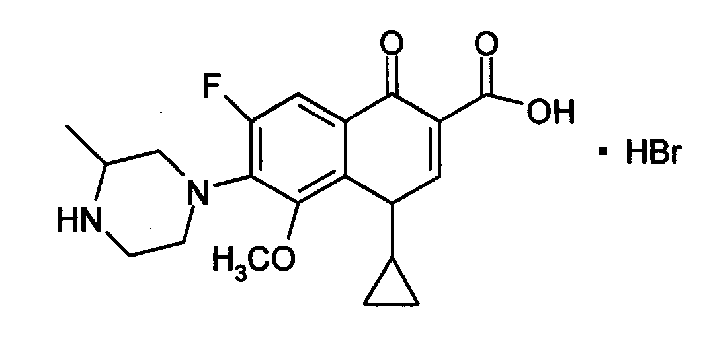
Gatifloxacin hydrobride as quinolone compound and its prepn and application as antibacterial agent
A technology of gatifloxacin and quinolones, applied in antibacterial drugs, organic chemistry, pharmaceutical formulations, etc., can solve problems such as unstable light exposure, easy yellowing of aqueous solutions, and achieve good product stability, good water solubility, Low irritant effect on human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Get 30ml of ethanol, 10 grams (0.027mol) of gatifloxacin, in a 250ml there-necked flask, stir and react at 45°C for 0.5 hour, add 6.5g (40%) (0.032mol) of hydrobromic acid, react at 50°C for 2 hours, drop to room temperature, suction filtered, washed with 10 ml of absolute ethanol × 2, and vacuum-dried to obtain 11 g of gatifloxacin hydrobromide.
Embodiment 2
[0019] Take 20ml of water, 10g (0.027mol) of gatifloxacin, in a 250ml there-necked flask, stir and react at 50°C for 0.5 hours, add 5.5g (40%) (0.027mol) of hydrobromic acid, react at 50°C for 2 hours, add acetone 100ml, cooled to room temperature, suction filtered, washed with 10ml of 90% acetone aqueous solution × 2, and vacuum-dried to obtain 10 grams of gatifloxacin hydrobromide.
Embodiment 3
[0021] 8.2 g (40%) (0.0405 mol) of hydrobromic acid, 30 ml of ethanol, and 10 g (0.027 mol) of gatifloxacin.
[0022] Get ethanol 30ml, gatifloxacin 10 grams (0.027mol), in 250ml there-necked bottle, 45 ℃ of stirring reaction 0.5 hour, add hydrobromic acid 8.2 gram (40%) (0.0405mol), 50 ℃ of reaction 2 hours, drop to room temperature, suction filtered, washed with 10 ml of absolute ethanol × 2, and vacuum-dried to obtain 10.8 g of gatifloxacin hydrobromide.
[0023] Tests have shown that the aqueous solution of the above-mentioned quinolone gatifloxacin hydrobromide dissolved in water basically does not change color under light conditions for 7 days.
[0024] Embodiment 3: antibacterial agent preparation for injection
[0025] Take an appropriate amount of quinolone gatifloxacin hydrobromide, and operate according to the preparation process procedures of injection, powder injection, and freeze-dried powder injection. During the preparation process, an appropriate amount of ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com