Method of preparing o-dihydroxybenzene using bionic catalytic oxygen to oxidize using bionic catalytic oxygen to oxidize phenol
A technology for oxidizing phenol and catechol, which is applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as high cost and complex reaction process, and achieve low reaction cost and synthetic operation Simple, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
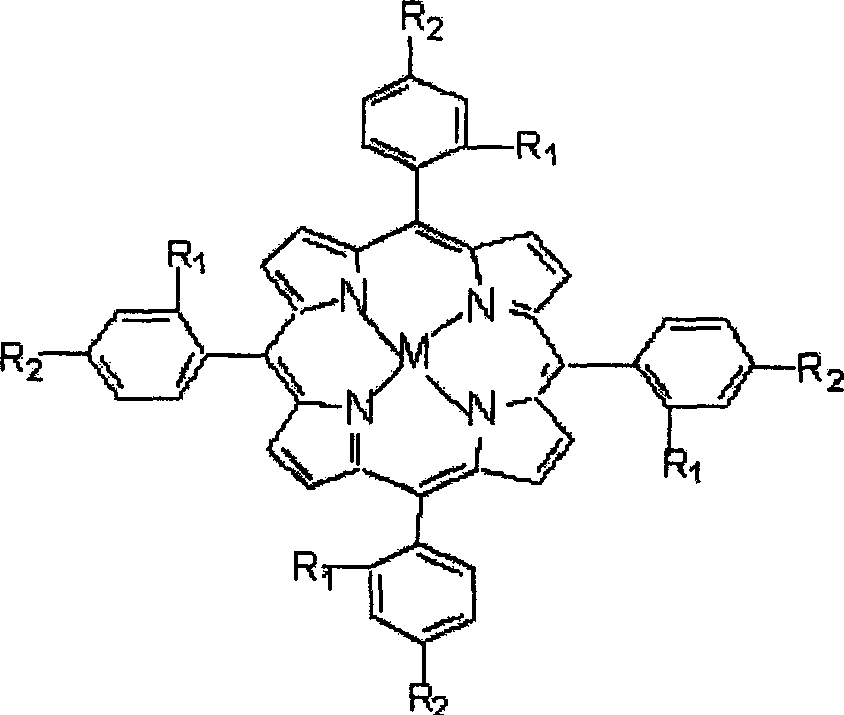
[0016] Take by weighing 11.28mg four-(o-chlorophenyl) cobalt porphyrin (being R in the general formula (I) 1 = Cl, R 2 =H, M=Co), 3.76g of phenol were put into a 100ml three-neck flask, 40ml of DMF was added, oxygen was introduced under normal pressure, and the mixture was magnetically stirred for 6 hours in a water bath with temperature control of 60°C. After the reaction was completed, the mixed solution was centrifuged to remove the catalyst, and the supernatant was taken, and analyzed and detected by gas chromatography. The conversion rate of phenol was 18.79%, and the selectivity of catechol was 81.41%.
Embodiment 2
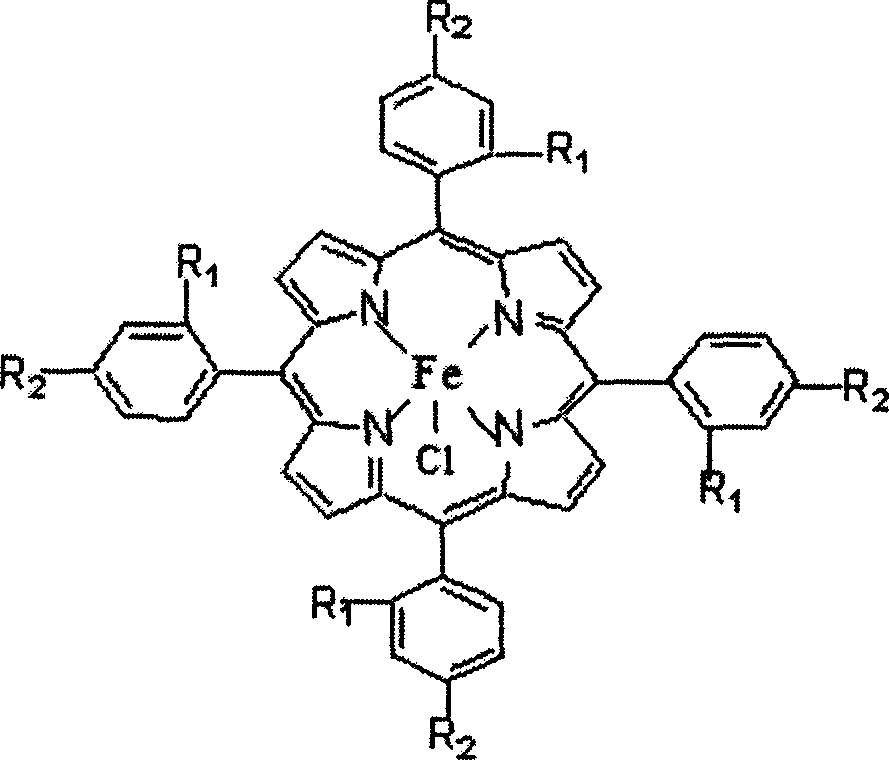
[0018] Take by weighing 22.56mg four-(o-chlorophenyl) iron porphyrin (being R in the general formula (I) 1 = Cl, R 2 =H, M=Fe), 3.76g of phenol, put into a 100ml three-neck flask, add 40ml of DMF, inject oxygen under normal pressure, and magnetically stir for 6h in a water bath with temperature control at 40°C. Subsequent processing steps are the same as in Example 1, and the conversion rate of phenol is 18.27% and the selectivity of catechol is 56.86% through gas chromatography analysis and detection.
Embodiment 3
[0020] Take by weighing 15.04mg four-(o-chlorophenyl) copper porphyrin (being R in the general formula (I) 1 = Cl, R 2 =H, M=Cu), 3.76g of phenol were put into a 100ml three-necked flask, 40ml of DMF was added, oxygen was introduced under normal pressure, and the mixture was magnetically stirred at 80°C in a water bath for 4h. Subsequent processing steps are the same as in Example 1, and the conversion rate of phenol is 16.16% and the selectivity of catechol is 78.55% through gas chromatography analysis and detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com