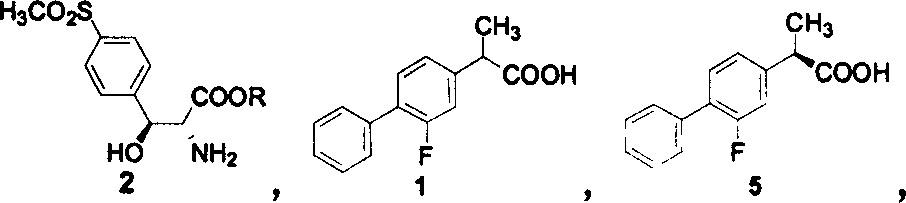
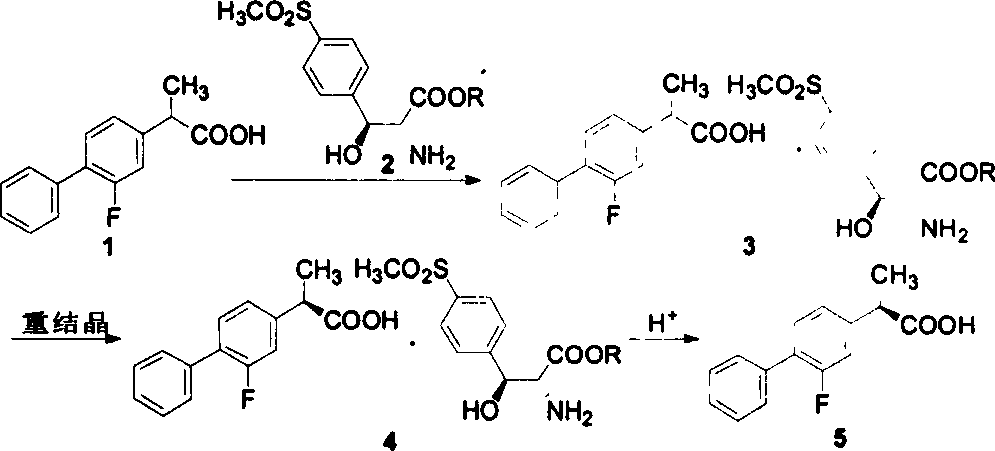
Preparation of levo flurbi profen
A technology of flurbiprofen and racemization, which is applied in the field of preparation of levoflurbiprofen, can solve the problems that the optical purity of levoflurbiprofen can only reach 73%, and is beneficial to industrial production, with mild reaction conditions, Effect of production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
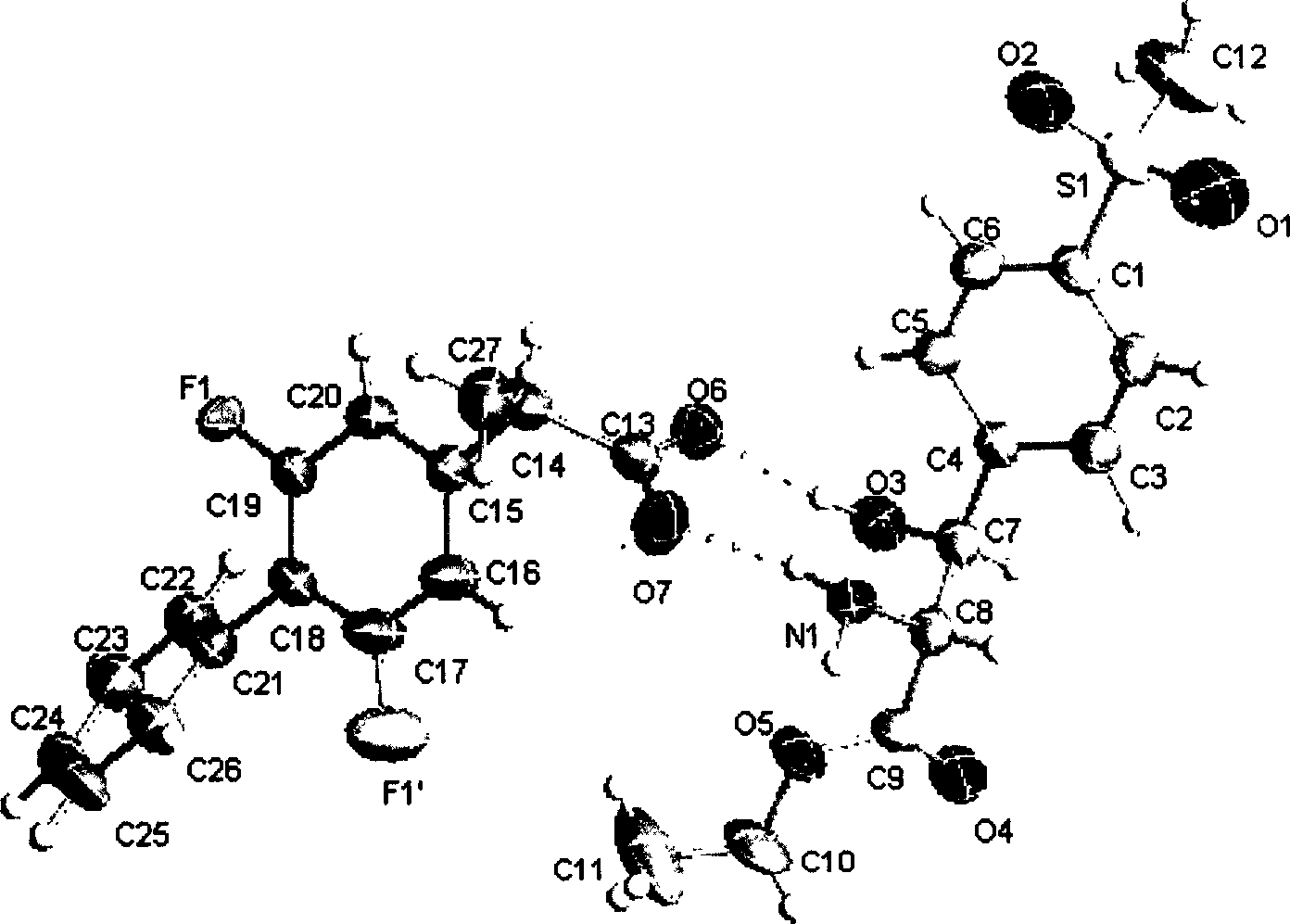
[0013] 234.3 grams of racemic flurbiprofen (purity 95%) were dissolved in 1515 milliliters of absolute ethanol, and 144.2 grams of (2R, 3S)-2-amino-3-hydroxyl-3-p-methylsulfone phenylpropionic acid ethyl ester was added (purity 91%), heated and co-flowed for 15 minutes, cooled to room temperature, precipitated crystals, filtered to obtain 227.4 grams of crude salt, and this crude salt was recrystallized twice with absolute ethanol to obtain 162.8 grams of pure product, its single crystal X- The ray spectrogram is shown in Figure 1. Suspend it in 470 ml of ethyl acetate, add 670 ml of 2N sulfuric acid aqueous solution, separate the layers, extract the inorganic phase with ethyl acetate (2 × 150ml), combine the organic phases, wash with 300 ml of 2N sulfuric acid aqueous solution, and then wash with water ( 4 × 200ml), dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 72.8 grams of crude acid, which was dissolved in 280 milliliters of glacial acetic acid, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com