Therapeutic agent to recover hematopoiesis function and its compositions and usage
A technology for hematopoietic function and composition, applied in the field of therapeutic agents, can solve the problems of reducing the effectiveness of toxic and side effects of the body's hematopoietic system, reducing the function of the body's bone marrow, and reducing the blood supply function, so as to reduce toxic reactions, reduce volume, and reduce toxic and side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] The 19 protein factors were divided into six groups and given to experimental animals. According to the understanding of the functions of these 19 protein factors, they were divided into six groups from A to F, as listed in Table 3. Each group of protein factors has similar or related functions of the same protein family. In experimental groups #2 to #7, protein combinations A to F were removed from the 19 protein factors to observe the recovery of the hematopoietic system by the corresponding protein combinations. The experimental results are summarized in Table 3.
[0166] table 3
[0167] In this experiment, one injection was administered, with 5 mice in each group. #8 is the control group, injected with phosphate buffered saline (PBS)
[0168] A FGF-1
[0169] Survival rate (%) 80 20 0 40 0 60 60 40
Embodiment 2
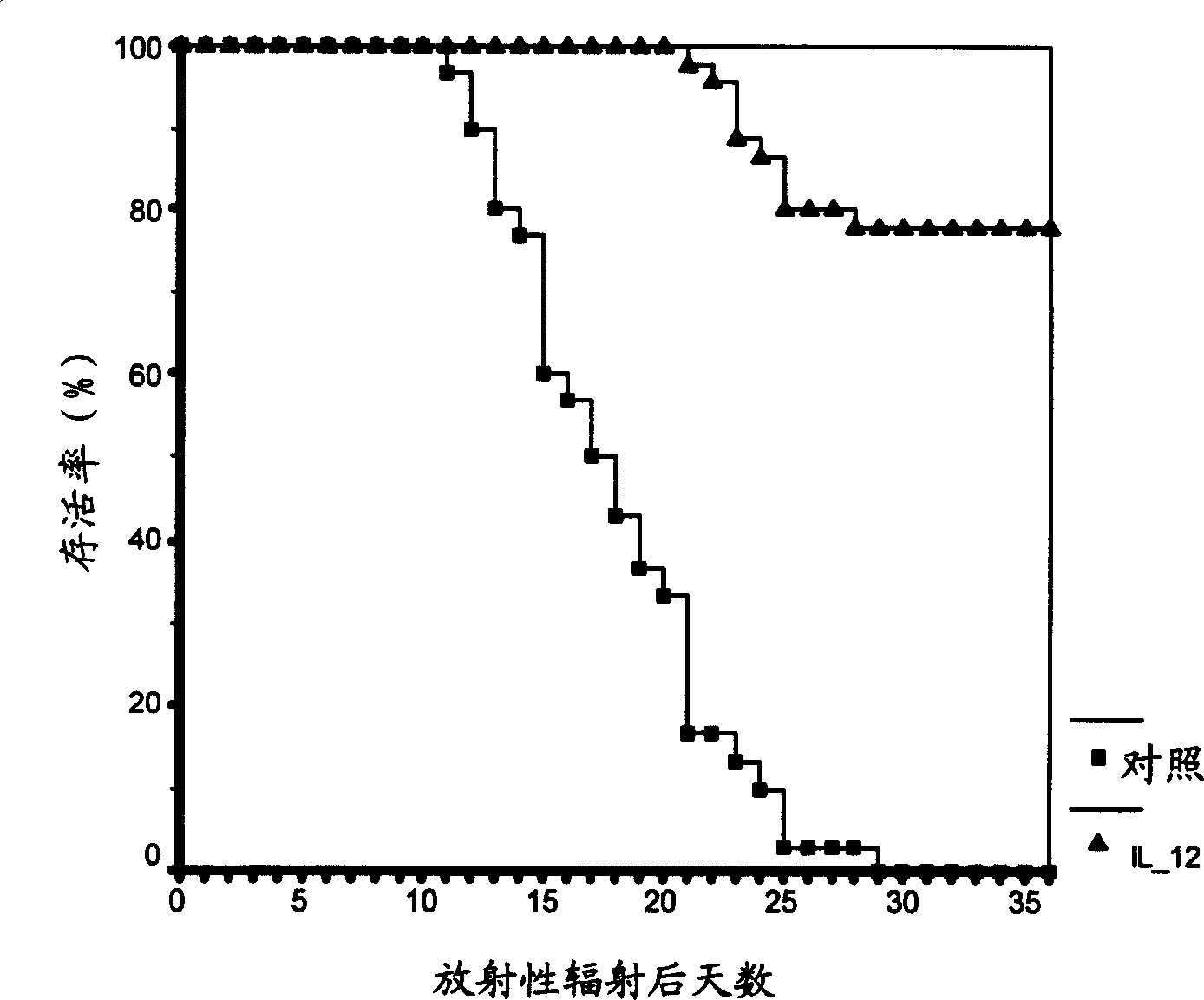
[0171] In this experiment, the mice were irradiated twice, 3 hours apart, 500 Rad each time. A single protein factor / formulation or a composition containing a single protein factor or a single protein factor plus serum was injected into mice once through the tail vein. Unless otherwise specified, the two administration time points are generally 30 to 60 minutes and 12 hours after the second radiation or 24 hours and 12 hours before the first radiation. The experimental results are summarized in figure 1 .
[0172] In Table 4 below, "after administration" means administering the composition 30 minutes and 12 hours after the second radiation; "after 30 minutes and 12 hours", "after 6 hours and 18 hours", "12 hours and After 24 hours", "after 24 hours and 36 hours" refers to administering the composition at a given time point after the second radiation; "24 hours and 12 hours before radiation" refers to 24 hours before the first radiation and administer the pharmaceutical comp...
Embodiment 3
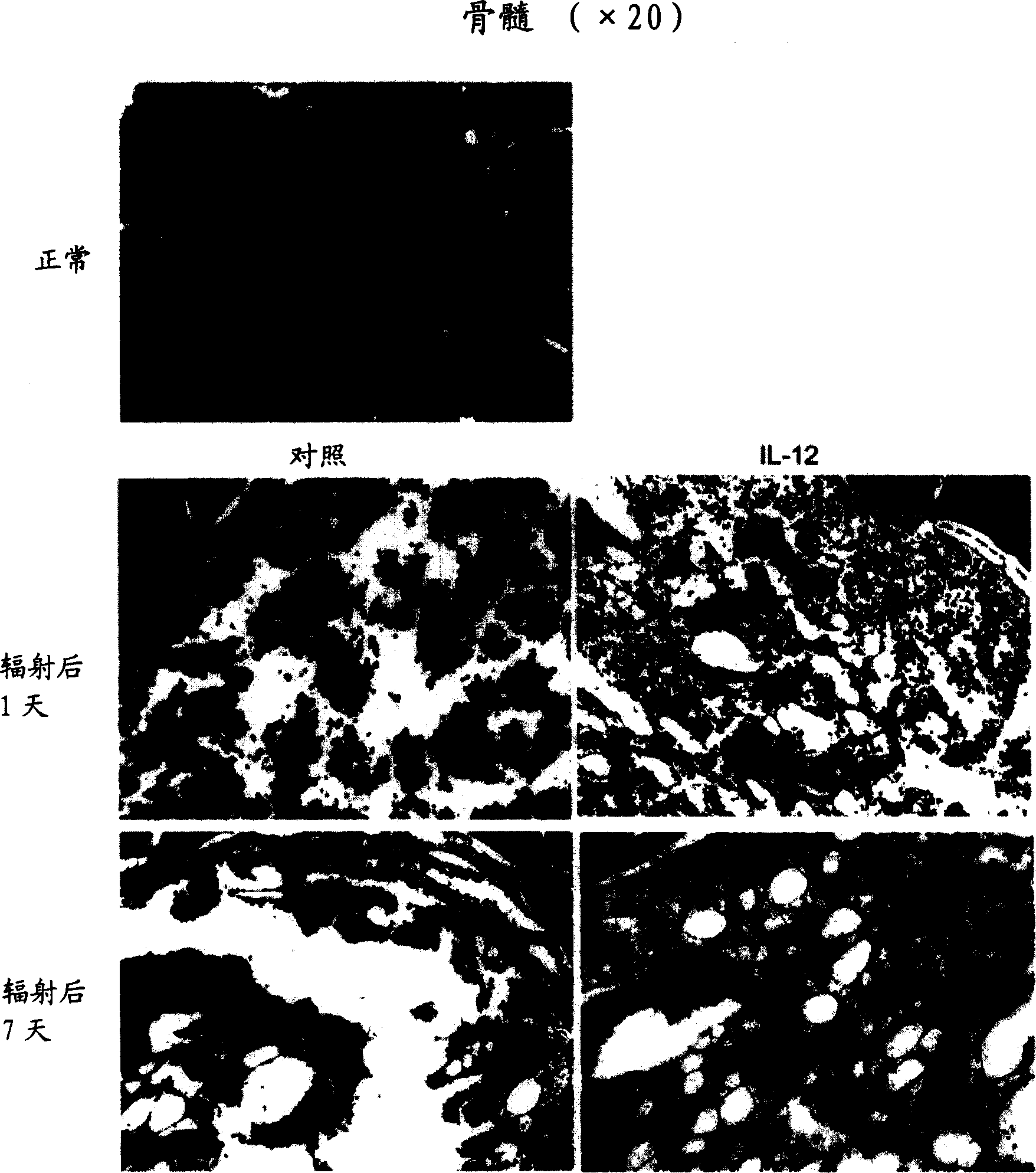
[0176] Histological studies were performed to show differences between control and experimental animals. In this experiment, 24 hours and 12 hours before the experimental mice received the second ionizing radiation radiation, IL-12 was injected into the tail vein of the mice respectively, with a dose of 100 ng per mouse each time, and mice in the control group were injected with Phosphate buffered saline (PBS buffer). After the injection, the mice in the control group and the experimental group received radiation radiation twice at the same time, with an interval of 3 hours in between, and each radiation dose was 500RAD. On the 1st and 7th day after radiation, the hind femurs were removed from the mice in the control combination experimental group, and fixed in 10% formalin for 24 hours for tissue sectioning and hematoxylin and eosin (H&E )dyeing. from figure 2 It can be seen that, compared with the control group, the protective effect of IL-12 on the bone marrow was clear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com