Method for stablizing protein medicine and its application in microball preparation
A protein drug and stabilization technology, which is applied in the preparation of microspheres, improves the stability of protein drugs, and can solve problems such as hidden dangers of formulation stability and decreased stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
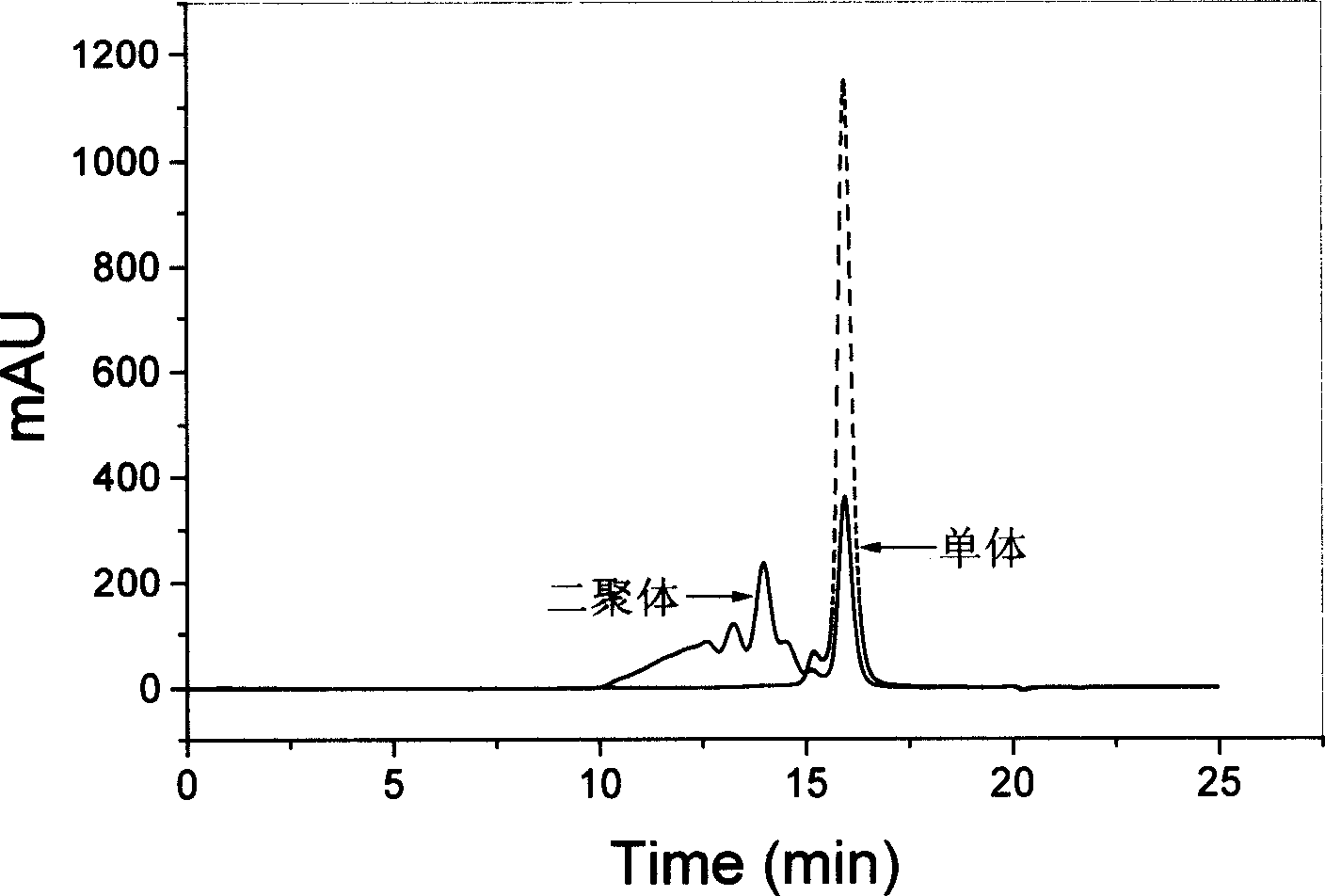
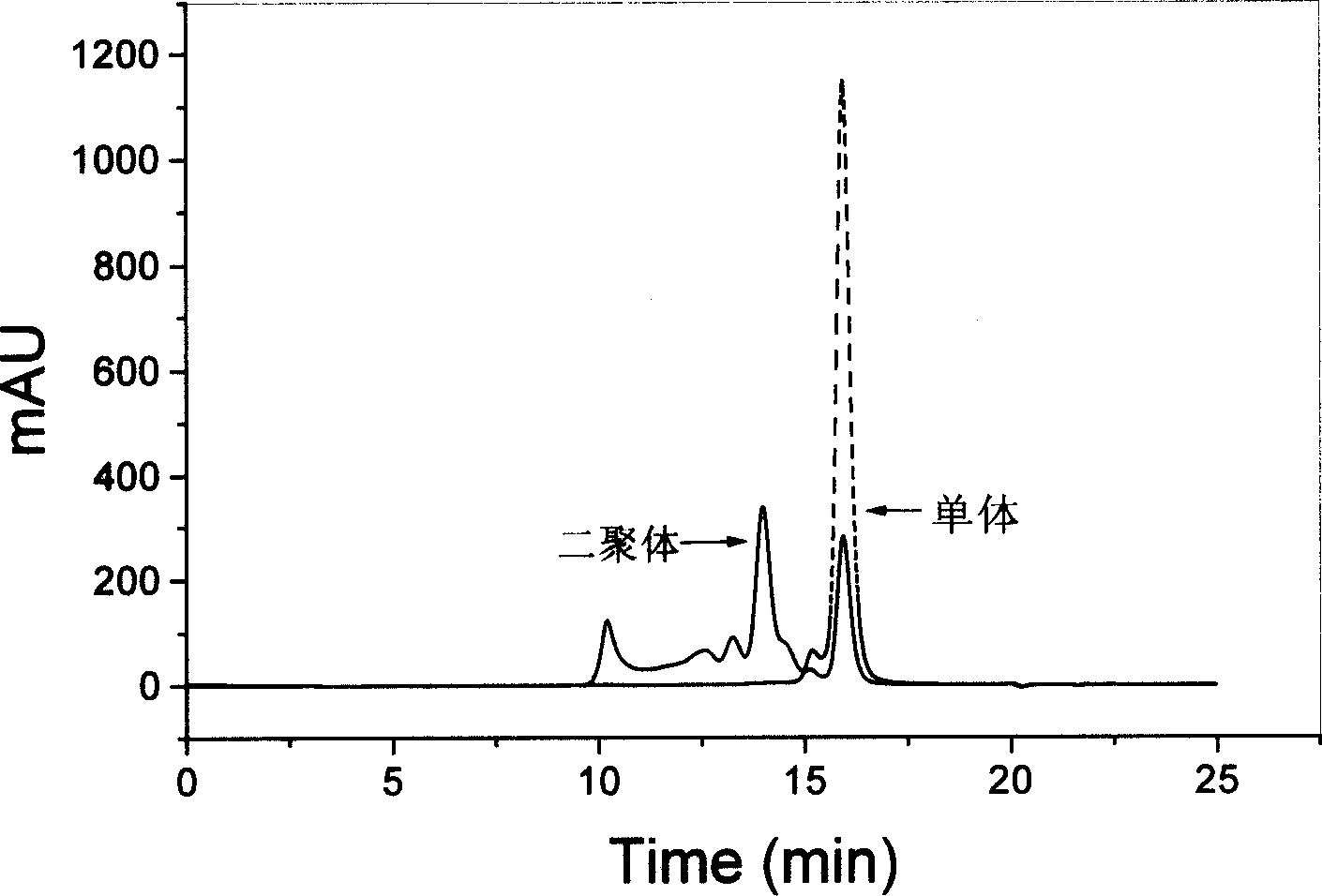
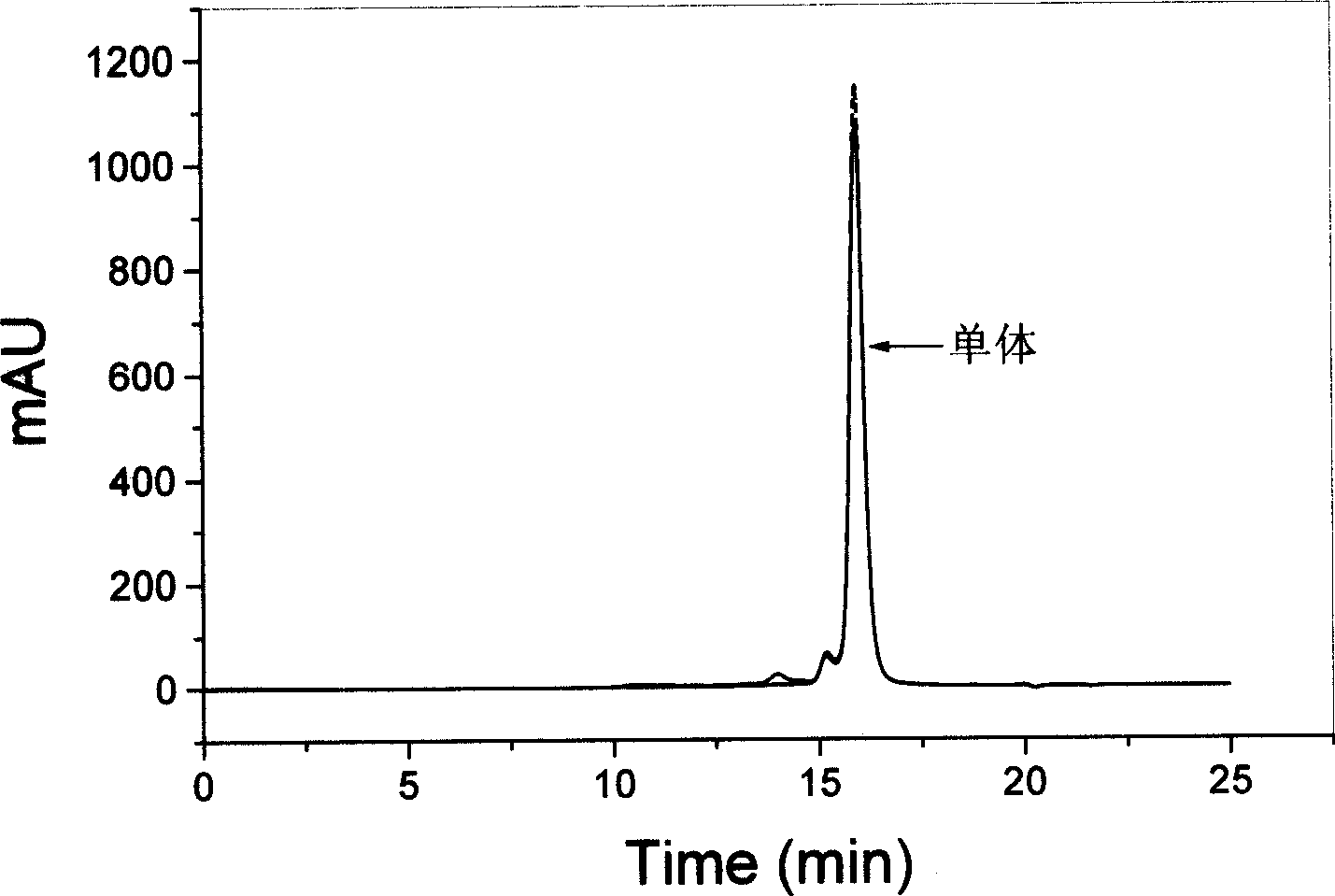
[0025] growth hormone stability test : Prepare a phosphate buffer solution (PBS, 10mM, pH8.0) containing various stabilizers and 50mg / ml recombinant human growth hormone (rhGH), mix the solution with 0.2ml of dichloromethane, stir vigorously or ultrasonically for 1min, Immediately transfer the water phase and repeatedly extract the oil phase with PBS (pH8.0) for 8 to 10 times, combine the extracts and dilute to 10ml. The solution was centrifuged at 4° C. and 3000 rpm for 30 min, and the supernatant was injected into a high-performance liquid chromatography system for determination.
[0026] The concentration of rhGH in the samples was determined by size exclusion chromatography (SEC). Chromatography system: Agilent1100series; Chromatographic column: TSK-Gel G2000SWXL (equipped with TSK-Guardcolumn SWXL pre-column); Column temperature: 25°C; Mobile phase: 3% isopropanol / 97% phosphate buffer solution (0.063M, pH7.0) ; Flow rate: 0.6ml / min; Detection wavelength: 214nm; Injecti...
Embodiment 2
[0047] Effect of Poloxamer Concentration on Growth Hormone Stability : In the PBS (10mM, pH8.0) solution that contains 50mg / ml rhGH, add the poloxamer 407 of different concentrations, this solution is mixed with dichloromethane as internal water phase, all the other operations and assay methods are with embodiment 1 . Table 2 is the effect of non-ionic surfactants on the stability of rhGH.
[0048] Table 2
[0049] High speed stirring Ultrasonic mixing
[0050] concentration
[0051] Stabilizer Soluble Protein Monomer Soluble Protein Monomer
[0052] mg / ml
[0053] % (a) % % %
[0054] control (b) 64.1 30.1 64.2 23.6
[0055] 2 57.9 34.2 63.1 33.6
[0056] Poloxamer 407 20 87.2 73.0 56.6 48.6
[0057] 50 97.5 90.0 97.0 89.1
[0058] 20 74.9 59.3 86.1 63.7
[0059] Tween 20 50 41.3 38.1 - (c) -
[006...
Embodiment 3
[0063] Preparation of Recombinant Human Growth Hormone Microspheres 1 : Dissolve 0.75 grhGH in 8 ml of PBS solution (10 mM, pH 8.0) containing 50 mg / ml poloxamer 407 and 45 mg / ml sucrose. This solution was gradually added dropwise to 20ml of dichloromethane solution containing 5g PLGA (75:25, MW 15kD). ), stirred at 1800rpm for 1min, poured the solution into 2L of distilled water and continued to stir at a lower speed for 4h to completely volatilize the organic solvent to obtain PLGA microspheres loaded with somatotropin. The obtained microspheres were collected by suction filtration on a suction filter, washed three times with distilled water and 0.2% poloxamer 188 solution, and dried in vacuum for 24 hours to obtain microsphere powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com