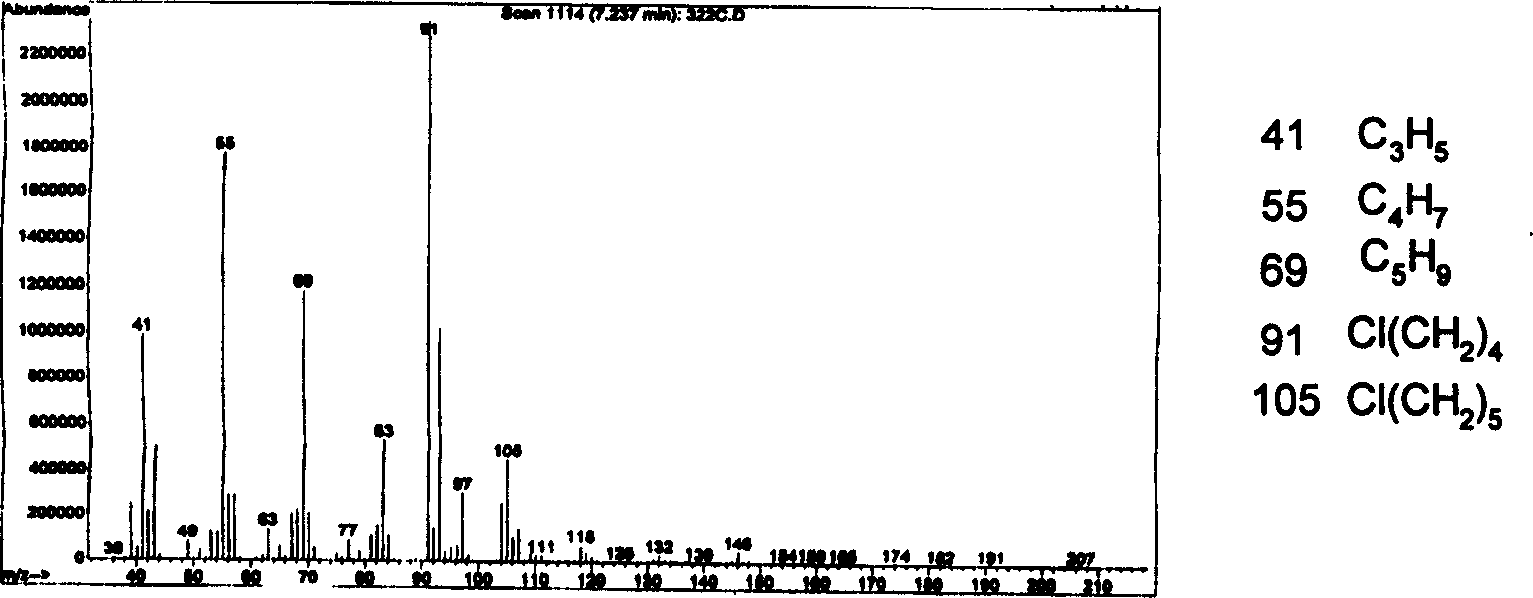
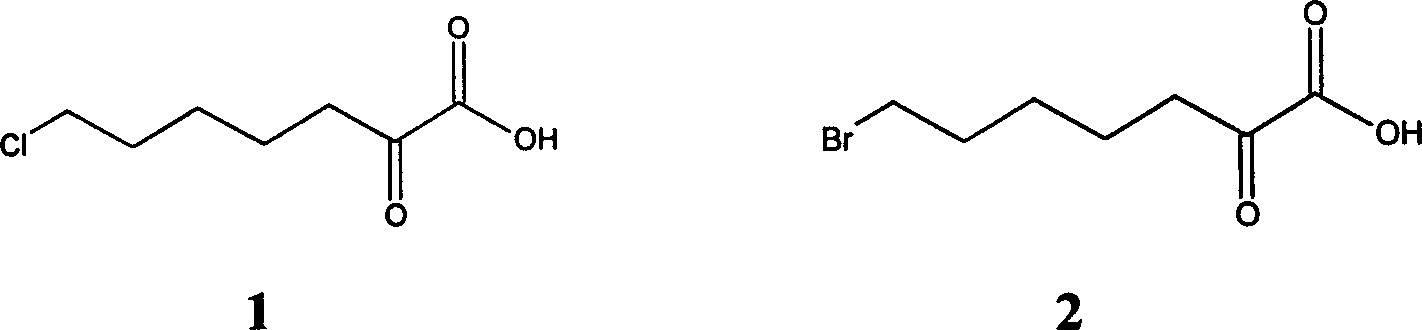
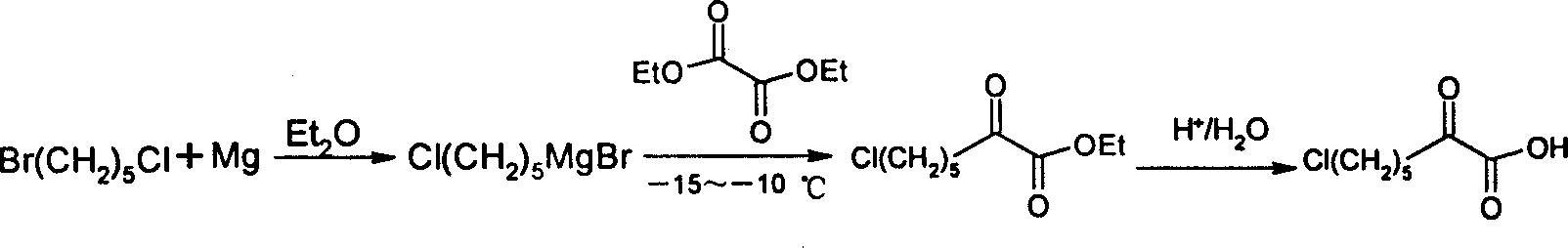
Process for synthesizing 7-chloro-2-oxo-heptanoic acid
A synthesis method and technology of ethyl oxoheptanoate, applied in the field of synthesis of 7-chloro-2-oxoheptanoic acid, can solve the problems of high cost, long steps, difficult raw materials and the like, and achieve low cost and reaction conditions. Gentle, simple-to-process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: A kind of synthetic method of 7-chloro-2-oxoheptanoic acid, using 1-bromo-5-chloro-pentane as the main starting material, is prepared through the following steps in sequence:
[0016] Step (1), first manufacture the single Grignard reagent of 1-bromo-5-chloro-pentane according to the conventional steps of Grignard reagent. Add 2.40 g (0.1 mol) of magnesium into the reactor, then add 10 ml of anhydrous ether and 0.2 ml of bromoethane, and stir to initiate the reaction. 18.6g (0.1mol) of 1-bromo-5-chloropentane was mixed with 70ml of anhydrous ether and then slowly added dropwise, the rate of addition was based on the slow reflux of ether. After dropping, heat the reaction until the magnesium powder is completely dissolved. The prepared single Grignard reagent is sealed and cooled for later use. Then carry out the addition reaction. Add 13.1 g (0.09 mol) of diethyl oxalate and 30 ml of anhydrous ether into a dry reaction flask, stir mechanically, protect ...
Embodiment 2
[0018] Embodiment two: a kind of synthetic method of 7-chloro-2-oxoheptanoic acid, take 1-bromo-5-chloro-pentane as main starting material, make through following steps successively:
[0019] Step (1), first manufacture the single Grignard reagent of 1-bromo-5-chloro-pentane according to the conventional steps of Grignard reagent. Add 2.88 g (0.12 mol) of magnesium into the reaction flask, then add 10 ml of anhydrous ether and 0.2 ml of ethyl bromide, and stir to initiate the reaction. 18.6g (0.1mol) of 1-bromo-5-chloropentane was mixed with 70ml of anhydrous ether and then slowly added dropwise, the rate of addition was based on the slow reflux of ether. After dropping, heat the reaction until the magnesium powder dissolves. The Grignard reagent is sealed and cooled for later use. Then carry out the addition reaction. Add 14.6 g (0.1 mol) of diethyl oxalate and 30 ml of anhydrous ether into a dry reaction flask, stir mechanically, protect with nitrogen, cool down to -15°C,...
Embodiment 3
[0021] Embodiment three: a kind of synthetic method of 7-chloro-2-oxoheptanoic acid, take 1-bromo-5-chloro-pentane as main starting material, make through following steps successively:
[0022] Step (1), first manufacture the single Grignard reagent of 1-bromo-5-chloro-pentane according to the conventional steps of Grignard reagent. Add 3.12 g (0.13 mol) of magnesium into the reaction flask, then add 10 ml of anhydrous ether and 0.2 ml of ethyl bromide, and stir to initiate the reaction. 18.6g (0.1mol) of 1-bromo-5-chloropentane was mixed with 70ml of anhydrous ether and then slowly added dropwise, the rate of addition was based on the slow reflux of ether. After dropping, heat the reaction until the magnesium powder dissolves. The Grignard reagent is sealed and cooled for later use. Then carry out the addition reaction. Add 16.1 g (0.11 mol) of diethyl oxalate and 30 ml of anhydrous ether into a dry reaction flask, stir mechanically, protect with nitrogen, cool down to -60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com