Method for separating and recovering sodium arsenate and alkali by fractional crystallization method
A technology for separation and recovery of sodium arsenate, which is applied in the field of separation and recovery of sodium arsenate and alkali, can solve the problems of difficult acceptance by manufacturers and high cost of acidification, and achieve the effects of simple equipment, increased concentration energy consumption, and significant environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
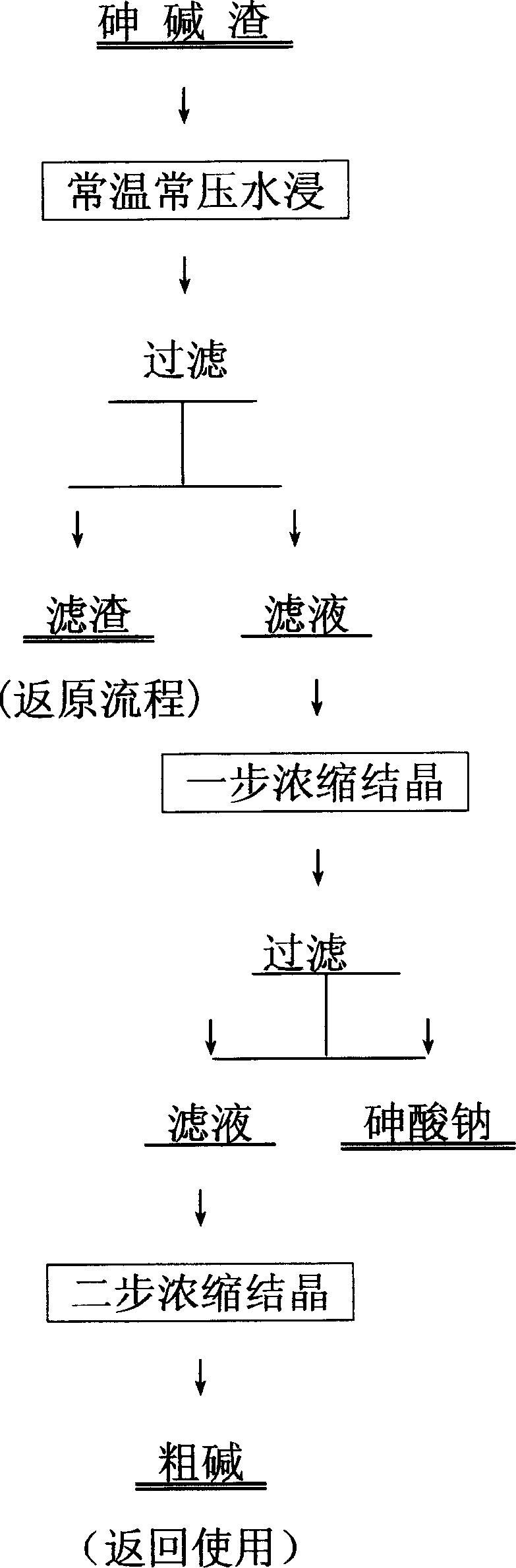
[0027] Example 1: 2 liters of As 5+ 32.46g / l, containing Na + 92.22g / l arsenic-alkali slag water immersion solution, concentrated at a temperature of 40-100°C to the end point of one-step crystallization, the solution contains As 5+ 65.32g / l, containing Na +203.00g / l, then cool the liquid to room temperature or below room temperature, let it stand for crystallization for 2 hours, and filter it to obtain Na 3 AsO 4 72.94%, containing NaOH1.34%, containing Na 2 CO 3 0.29%, containing H 2 O25.44% sodium arsenate crystal 220g. The direct recovery rate of As is 89.13%, and the recovery rate is 97.60%. Then put the one-step crystallization mother liquor in the concentrator, concentrate at 40-100°C until the second-step crystallization reaches a water content of 15-30%, then discharge it from the concentrator, put it into the furnace for calcination, and obtain 220 g of calcined products, containing NaOH82. 16%, Na 2 CO 3 10.27%, containing Na 3 AsO 4 6.93%. Contains H ...
Embodiment 2
[0028] Embodiment 2: 2 liters containing As 5+ 38.22g / l, containing Na + 93.22g / l arsenic-alkali slag water immersion solution, concentrated at a temperature of 40-100°C to the end point of one-step crystallization, the solution contains As 5+ 62.36g / l, containing Na + 196.30g / l, then cool the liquid to room temperature or below room temperature, let it stand for crystallization for 2 hours, and filter it to obtain Na 3 AsO 4 77.65%, containing NaOH1.36%, Na 2 CO 3 1.06%, containing H 2 O19.93% sodium arsenate crystals 246g. The direct recovery rate of As is 90.11%, and the recovery rate is 96.07%. Then put the one-step crystallization mother liquor in the concentrator, concentrate at 40-100°C until the second-step crystallization reaches a water content of 15-30%, then discharge it from the concentrator, put it into the furnace for calcination, and obtain 209g of calcined product, containing NaOH83. 49%, Na 2 CO 3 8.96%, containing Na 3 AsO 4 6.05%. Contains H ...
Embodiment 3
[0029] Embodiment 3: 4 liters containing As 5+ 32.39g / l, containing Na + 88.32g / l arsenic-alkali slag water immersion solution, concentrated at a temperature of 40-100°C to the end point of one-step crystallization, the solution contains As 5+ 69.33g / l, containing Na + 190.31g / l, then cool the liquid to room temperature or below room temperature, let it stand for crystallization for 2 hours, and filter it to obtain Na 3 AsO 4 63.81%, containing NaOH6.83%, Na 2 CO 3 0.31%, containing H 2 O2 9.05% sodium arsenate crystal 501g. The direct recovery rate of As is 88.98%, and the recovery rate is 97.22%. Then put the one-step crystallization mother liquor in the concentration equipment, concentrate at 40-100°C until the second-step crystallization product reaches a water content of 15-30%, then discharge it from the concentration equipment, put it into the furnace for calcination, and obtain 403g of calcined product, containing NaOH79. 11%, Na 2 CO 3 12.36%, containing Na ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com