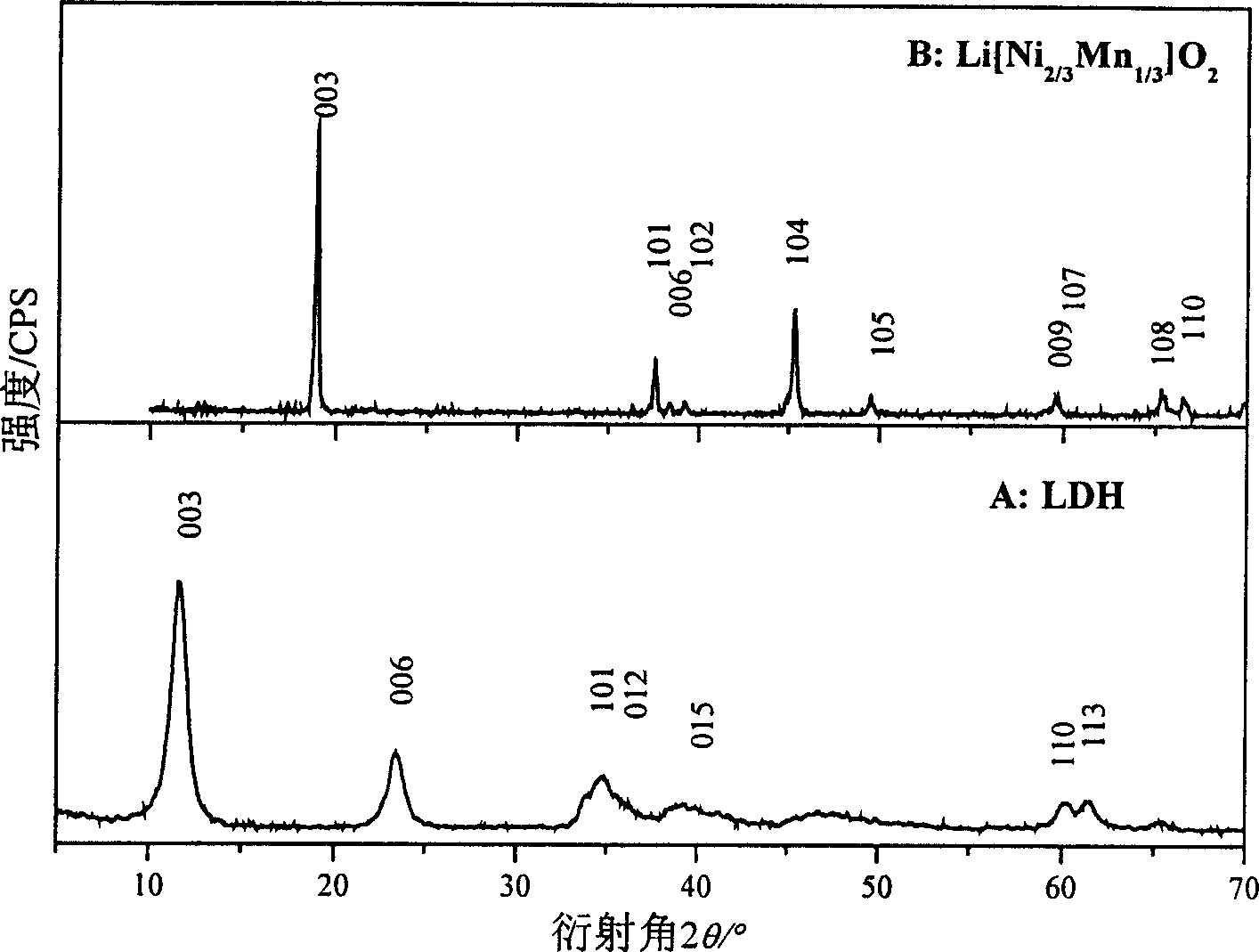
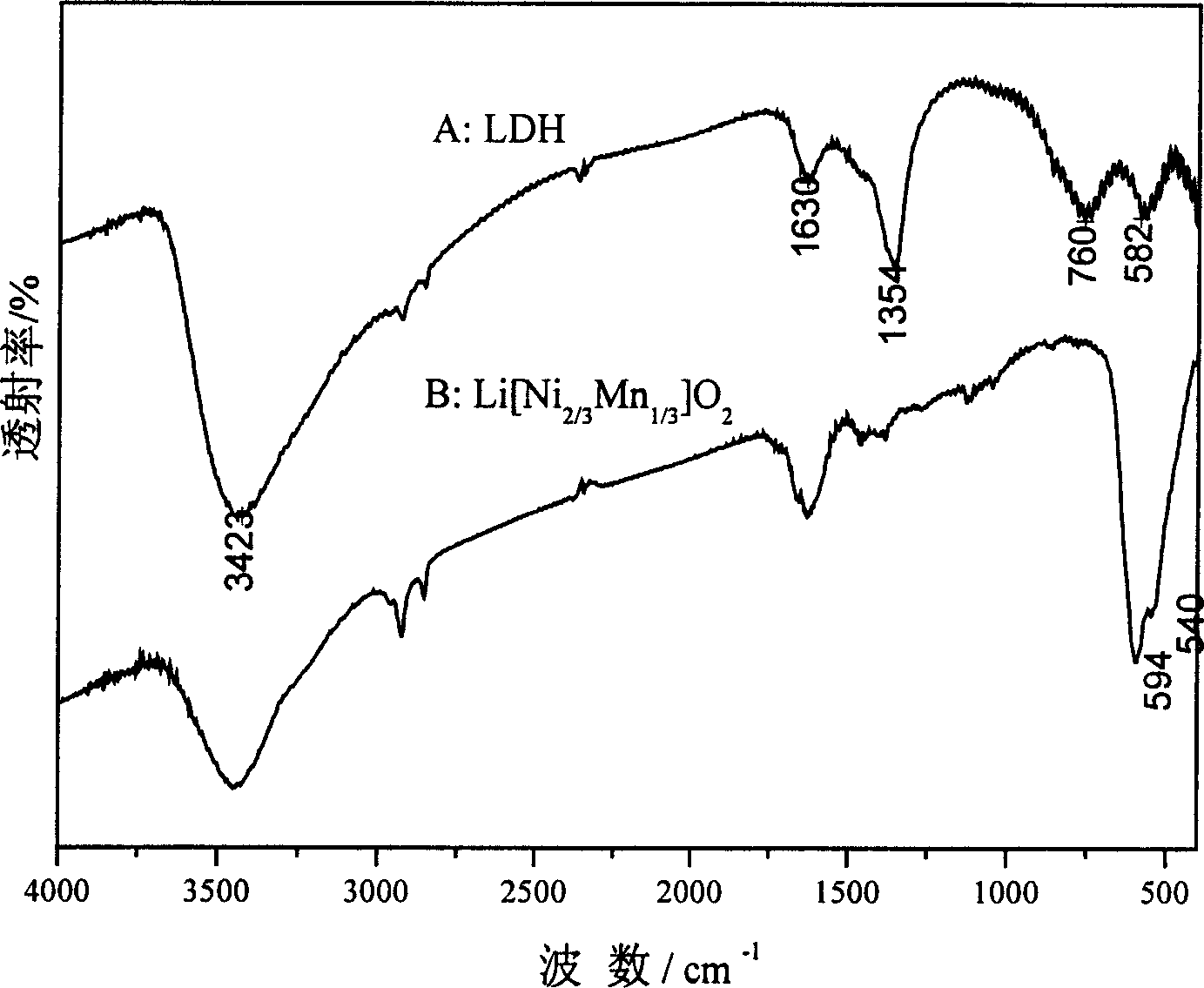
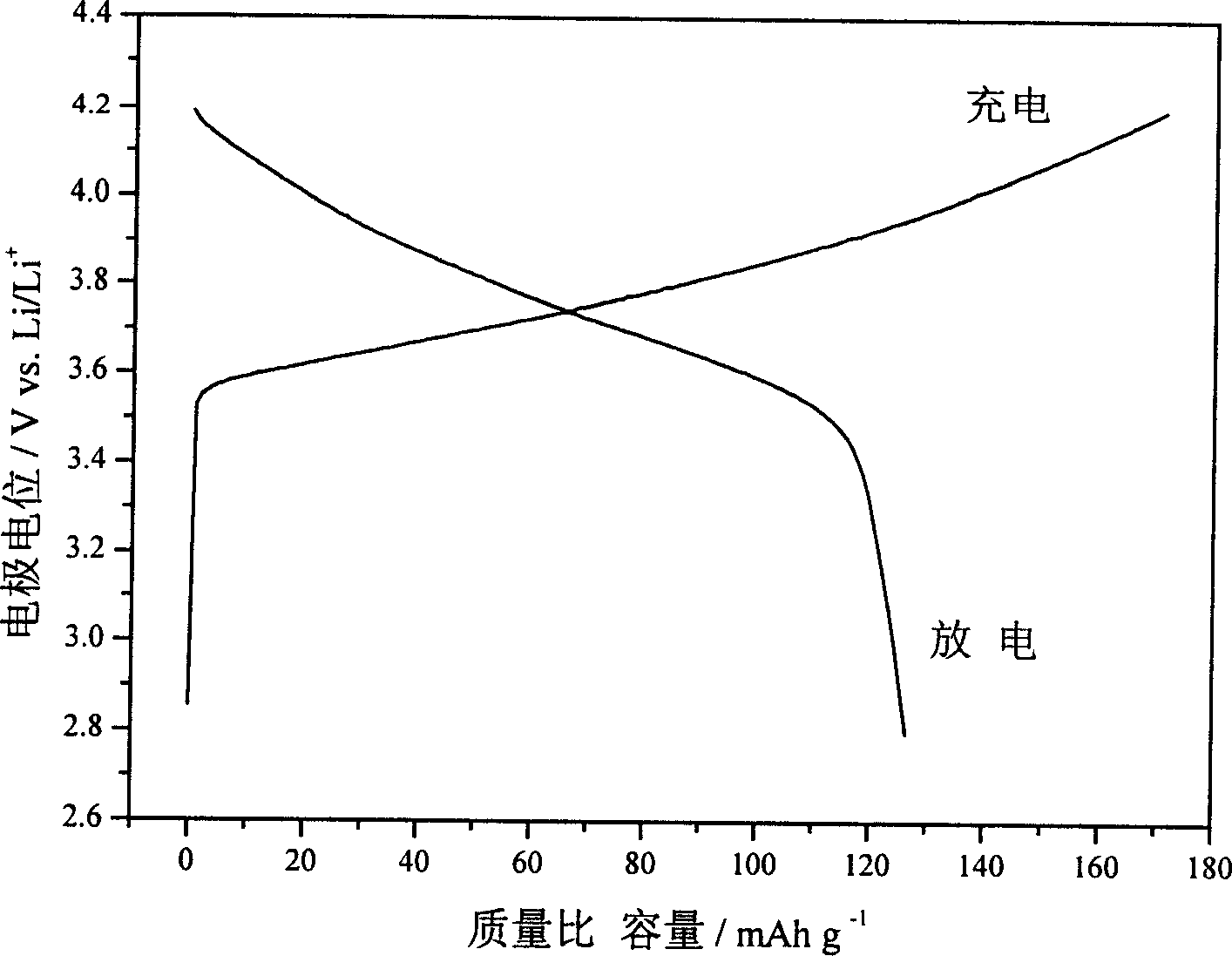
Lithium ion battery positive pole material layered transition metal composite oxide and method of preparation
A cathode material layer, lithium-ion battery technology, applied in the directions of alkali metal compounds, lithium compounds, electrode manufacturing, etc., can solve the problems of electrochemical specific capacity decay, slow electrochemical specific capacity decay, easy to change and other problems, achieve layered Complete structure, good cycle performance, easy operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1). Accurately weigh 0.04mol of Ni(NO 3 ) 2 ·6H 2 O and 0.02mol Mn(NO 3 ) 2 50% aqueous solution of 100mL of water to form a mixed salt solution A with a Ni / Mn molar ratio equal to 2; another accurately weigh 0.1mol of NaOH and 0.05mol of NaOH 2 CO 3 , use 100mL water to make mixed alkali solution B;
[0027] (2). Slowly drop the mixed alkali solution B into the vigorously stirred mixed salt solution A under air flow conditions for about 5 hours; the resulting slurry was crystallized at 30°C for 10 hours, centrifuged and washed to obtain Ni- Mn LDH wet cake;
[0028] (3). Weigh 0.066mol of LiOH·H 2 O (Li / Ni+Mn molar ratio is 1.1), add 10mL water to dissolve, mix evenly with LDH wet filter cake, dry at 70°C for 12h, then roast at 750°C for 24h, heating rate 10°C / min, and cool down to room temperature naturally, After calcination, the sample was fully washed with water until the pH was 7, and dried at 70 °C for 24 h to obtain a layered Li[Ni 0.67 mn 0.33 ]O 2 ...
Embodiment 2
[0034] (1). Accurately weigh 0.048mol of Co(NO 3 ) 2 ·6H 2 O and 0.012mol Mn(NO 3 ) 2 50% aqueous solution of 100mL of water to form a mixed salt solution A with a Ni / Mn molar ratio equal to 2; another accurately weigh 0.1mol of NaOH and 0.05mol of NaOH 2 CO 3 , use 100mL water to make mixed alkali solution B;
[0035] (2). Slowly drop the mixed alkali solution B into the vigorously stirred mixed salt solution A under air flow conditions for about 5 hours; the resulting slurry is crystallized at 30°C for 10 hours, centrifuged and washed to obtain Co- Mn LDH wet cake;
[0036] (3). Weigh 0.066mol of LiOH·H 2 O (Li / Co+Mn molar ratio is 1.1), add 10mL water to dissolve, mix evenly with LDH wet filter cake, dry at 70°C for 12h, then roast at 750°C for 24h, heating rate 10°C / min, naturally cool down to room temperature, After calcination, the sample was fully washed with water until the pH was 7, and dried at 70 °C for 24 h to obtain a layered Li[Co 0.80 mn 0.20 ]O 2 . ...
Embodiment 3
[0041] (1). Accurately weigh 0.015mol of Ni(NO 3 ) 2 ·6H 2 O, 0.03mol of Co(NO 3 ) 2 ·6H 2 O and 0.015mol Mn(NO 3 ) 2 50% aqueous solution of 100mL water to make a mixed salt solution A with a Ni / Co / Mn molar ratio equal to 1 / 2 / 1; another accurately weigh 0.1mol of NaOH and 0.05mol of NaOH 2 CO 3 , use 100mL water to make mixed alkali solution B;
[0042] (2). Slowly drop the mixed alkali solution B into the vigorously stirred mixed salt solution A under air flow conditions for about 5 hours; the resulting slurry was crystallized at 30°C for 10 hours, centrifuged and washed to obtain Ni- Co-Mn LDH wet cake;
[0043] (3). Weigh 0.066mol of Li 2 CO 3 (Li / Ni+Co+Mn molar ratio is 1.1), add 10mL water to dissolve, mix evenly with LDH wet filter cake, dry at 70°C for 12h, then roast at 800°C for 24h, heating rate 10°C / min, and cool down to room temperature naturally After calcination, the sample was fully washed with water until the pH was 7, and dried at 70°C for 24 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com