Halogen-blended process of preparing low-sodium carnallite
A carnallite and brine technology, which is applied in the direction of magnesium halide, alkali metal chloride, magnesium chloride, etc., can solve the problems of high cost of low-sodium carnallite, increased consumption of bischofolite, complex process, etc. Achieve the effects of high added value, convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
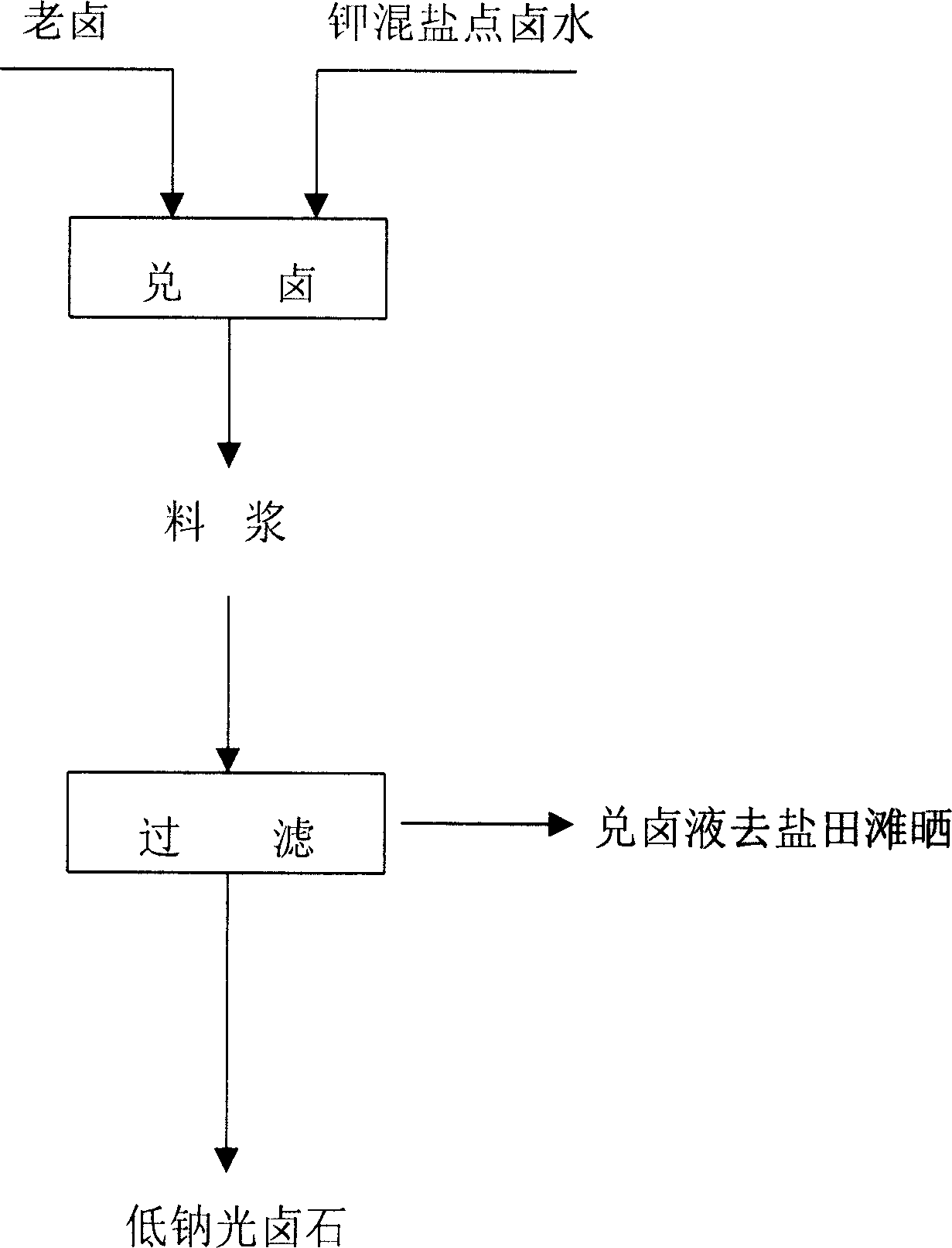
[0013] Example 1. A method for preparing low-sodium carnallite by mixing brine. The collected magnesium sulfate subtype brine is sunned in salt fields and naturally evaporated to precipitate solid salt, so that the magnesium ions Mg in the brine ++ The percentage content is 7.0% to obtain the old brine; then the old brine and potassium mixed salt point brine are subjected to a crystallization reaction with brine at a weight ratio of 2:1, and filtered to obtain low-sodium carnallite.
Embodiment 2
[0014] Example 2. A method for preparing low-sodium carnallite by mixing brine, in which the collected magnesium sulfate subtype brine is dried on the beach in salt fields, and naturally evaporated to precipitate solid salts, sodium chloride NaCl, and epsom salt MgSO 4 .7H 2 O, langbeinite K 2 SO 4 .MgSO 4 .6H 2 O, kainite KCl.MgSO 4 .3H 2 O, carnallite KCl.MgCl 2 .6H 2 After O etc., the magnesium ions Mg in the brine ++ The percentage content is 8.0%, and the old brine is obtained; then the old brine and potassium mixed salt point brine are added to the brine mixing container at a weight ratio of 1:1, and the full mixed flow is continuously stirred at a temperature of 10°C for 0.5 hours, and then filtered. To obtain low-sodium carnallite, the brine obtained after filtration is returned to the salt field for drying. Potassium ion K in the potassium mixed salt point brine described in this embodiment + The percentage content is 0.5%, magnesium ion Mg ++ The percenta...
Embodiment 3
[0015] Example 3. A method for preparing low-sodium carnallite by mixing brine, in which the collected magnesium sulfate subtype brine is dried on the beach in salt fields, and naturally evaporated to precipitate solid salts, sodium chloride NaCl, and epsom salt MgSO 4 .7H 2 O, langbeinite K 2 SO 4 .MgSO 4 .6H 2 O, kainite KCl.MgSO 4 .3H 2 O, carnallite KCl.MgCl 2 .6H 2 After O etc., the magnesium ions Mg in the brine ++ The percentage content is 9.0% to obtain the old brine; then add the old brine and potassium mixed salt point brine into the brine mixing container at a weight ratio of 3:1, carry out full-mixed continuous stirring at a temperature of 40 C for 2 hours, and filter to obtain For low-sodium carnallite, the brine solution obtained after filtration is returned to the salt field for drying. Potassium ion K in the potassium mixed salt point brine described in this embodiment + The percentage content is 0.6%, magnesium ion Mg ++ The percentage content is 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com