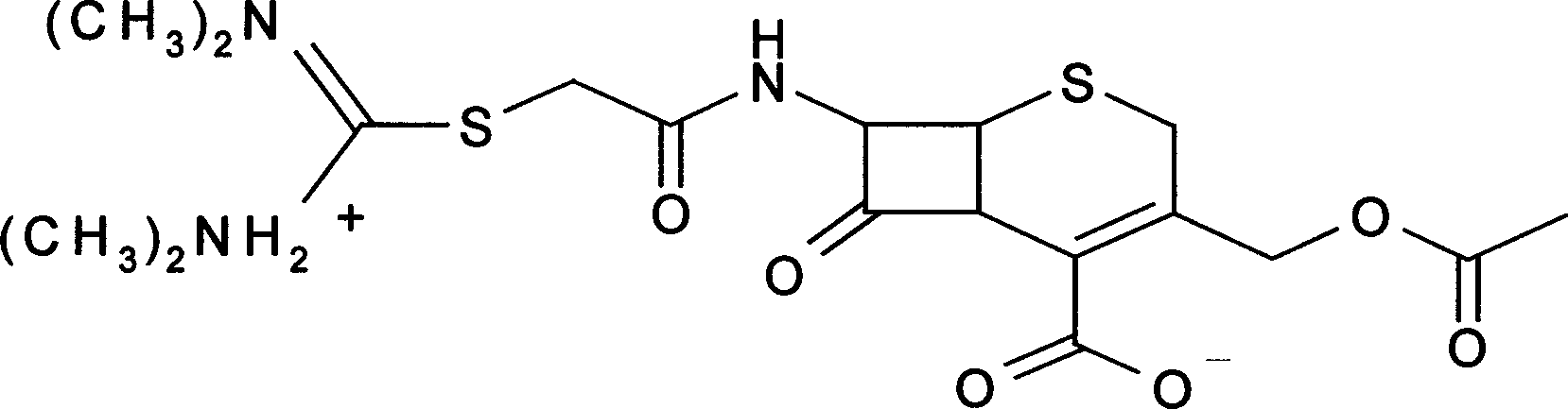
Method for preparing cefathiamidine freeze-dried sterile raw material medicine
A technology of cefathiamidine and raw material medicine, which is applied in the field of preparation of cefathiamidine freeze-dried sterile raw material medicine, can solve the problems of increasing production cost, affecting product quality, low yield, etc., and achieves low production cost, transportation or storage The effect of convenience and stable drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Accurately weigh 50g of non-sterile cefathiamidine (prepared by the method in US Pat. After 20 minutes, perform sterile filtration, transfer the resulting filtrate to a freeze-drying tray, and freeze-dry according to the freeze-drying process conditions. In the final stage of freeze-drying, when the temperature is raised to about 25°C, the vacuum degree does not change within 1 hour. 48.8g of the product was prepared, the water content was 2.5%, and the yield was 97.6%.
Embodiment 2
[0024] Accurately weigh 50g of non-sterile cefathiamidine (prepared by the method in U.S. Patent No. 3,646,025), dissolve it in 500 ml of water for injection under aseptic, room temperature and mechanical stirring, and add 2g of activated carbon for decolorization After 25 minutes, perform sterile filtration, transfer the resulting filtrate to a freeze-drying tray, and freeze-dry according to the freeze-drying process conditions. In the final stage of freeze-drying, when the temperature is raised to about 25°C, the vacuum degree does not change within 3 hours. 49.0 g of the product was prepared, the water content was 1.8%, and the yield was 98.0%.
Embodiment 3
[0026] Accurately weigh 50 g of non-sterile cefathiamidine (prepared by the method in U.S. Patent No. 3,646,025), dissolve it in 500 ml of water for injection under aseptic, room temperature, and mechanical stirring, and add 1 g of activated carbon for decolorization After 30 minutes, perform sterile filtration, transfer the resulting filtrate to a freeze-drying tray, and freeze-dry according to the freeze-drying process conditions. In the final stage of freeze-drying, when the temperature is raised to about 25°C, the vacuum degree does not change within 5 hours. 49.2 g of the product was prepared, the water content was 0.7%, and the yield was 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com