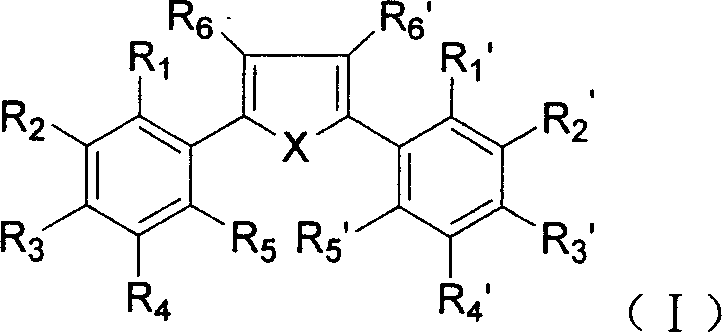
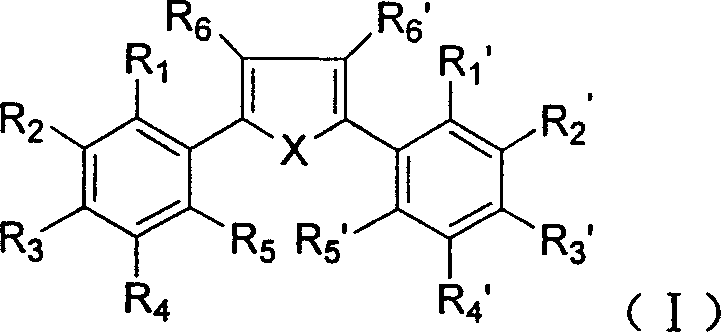
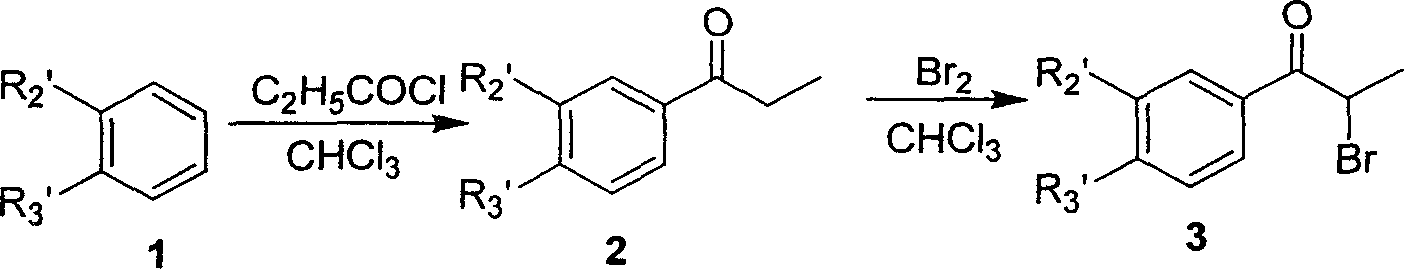
Dialkyl and diaryl tetrasubstituted heterocyclic compounds, preparing process and use thereof
A kind of heterocyclic compound and tetra-substituted technology, which is applied in the field of preparation of diaryl tetra-substituted heterocyclic compounds, dialkyl, and can solve problems such as dialkyl that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Synthesis of 2-(3-bromo-4-methoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-3,4-dimethylthiophene
[0098] (A) Preparation of o-bromoanisole (1)
[0099] In a round bottom flask equipped with a reflux condenser and a magnetic stirring device, 60 ml of 37% KOH aqueous solution and 50 ml of o-bromophenol were added at room temperature. The mixture was heated to 85°C with sufficient stirring and kept for 0.5 hours, and then sulfuric acid was added dropwise. 41ml of methyl ester, keep the pH of the reaction solution> 8, and continue the reaction for 48 hours. After the reaction was completed, it was extracted with ether, the organic layer was washed with a 10% KOH aqueous solution, and the solvent was evaporated to obtain a pale yellow liquid o-bromoanisole with a yield of 86%.
[0100] (B) Preparation of 3-bromo-4-methoxypropiophenone (2)
[0101] Add 200ml of dried CHCl to a dry round bottom flask 3 And 86g AlCl 3 , Keep the temperature of the reaction solution at 0~5℃ with an ice wa...
Embodiment 2
[0118] Preparation of 2-(3-bromo-4-methoxyphenyl)-3,4-dimethyl-5-(3,,4,5-trimethoxyphenyl)furan (9)
[0119] In a round bottom flask equipped with a reflux device, 100 mg of 1-(3-bromo-4-methoxyphenyl)-2,3-dimethyl-4-(3,,4,5-trimethoxy Phenyl)-1,4-butanedione (7) dissolved in 2mlCH 2 Cl 2 In the solution, and add 2ml HCl methanol solution (made by adding 5ml concentrated hydrochloric acid to 100ml methanol solution). After the reaction mixture was heated to reflux for 1 hour, TLC detected that the reaction was complete. Crystals separated out after cooling, filtered to obtain 85 mg of white crystals, which were determined to be 2-(3-bromo-4-methoxyphenyl)-3,4-dimethyl-5-(3,,4,5- Trimethoxyphenyl) furan, the yield was 88.5%.
[0120] 1 HNMR(CDCl 3 )δppm 2.17~2.22(m, 6H, -CH 3 ), 3.86~3.91(m, 12H, OCH 3 ), 6.84-7.83 (m, 5H, ArH).
Embodiment 3
[0122]Preparation of 2-(3-bromo-4-methoxyphenyl)-3,4-dimethyl-5-(3,,4,5-trimethoxyphenyl)-1H-pyrrole (10).
[0123] Add 55 mg of 1-(3-bromo-4-methoxyphenyl)-2,3-dimethyl-4-(3,,4,5-trimethoxyphenyl)-1,4-butanedione (7) Dissolve in 0.7ml EtOH and 1ml CHCl 3 In the solution, heat and reflux for 10 hours. After cooling the reaction solution, the solvent was removed under reduced pressure to obtain a crude product, which was separated by silica gel column (ethyl acetate / petroleum ether = 1 / 4) to obtain a white solid, which was determined to be 2-(3-bromo-4-methoxybenzene) Yl)-5-(3,4,5-trimethoxyphenyl)-3,4-dimethylpyrrole, the yield was 75.8%.
[0124] 1 HNMR(DMSO)δppm 2.05(s, 3H, -CH 3 ), 2.11(s, 3H, -CH 3 ), 3.65(s, 3H, -OCH 3 ), 3.80(s, 6H, OCH 3 ), 3.84(s, 3H, OCH 3 ), 6.74-7.66 (m, 5H, ArH), 10.59 (s, 1H, -NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com