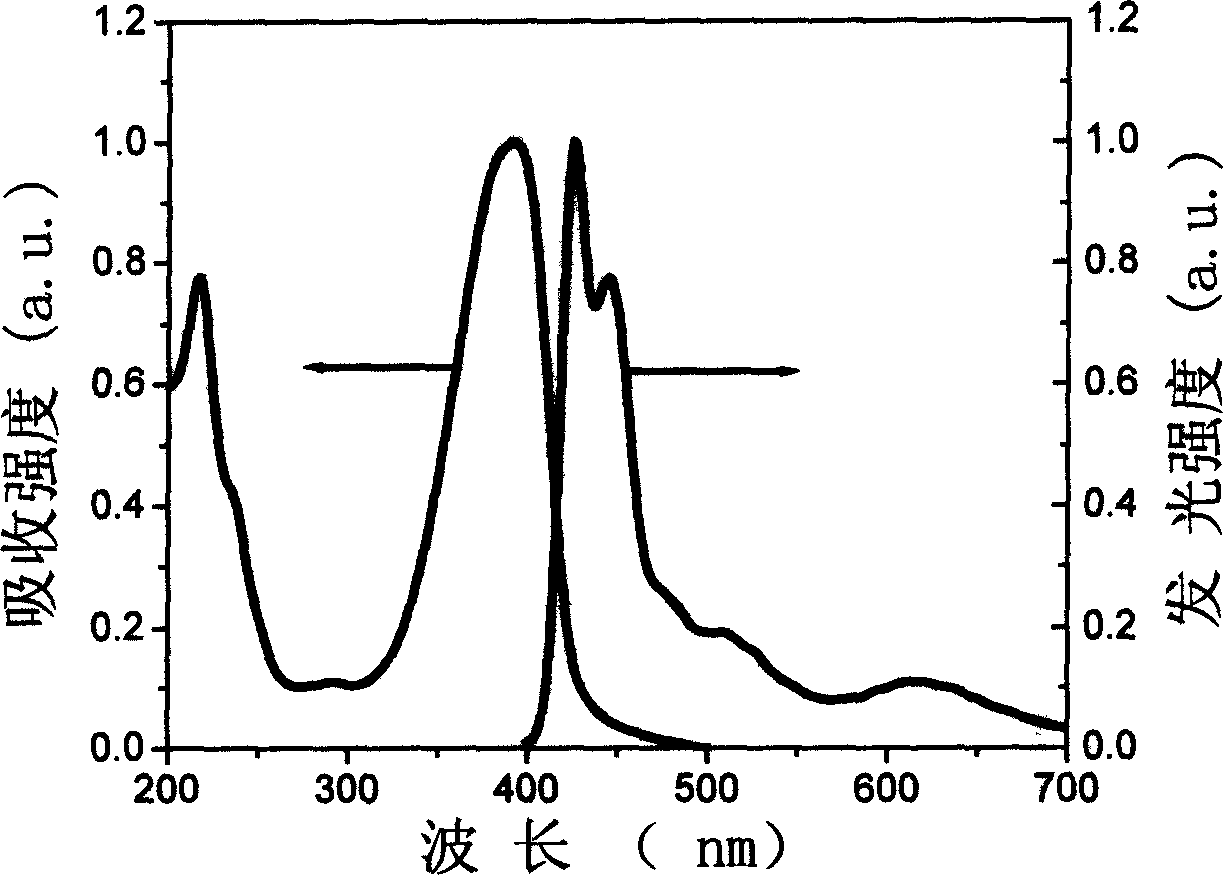
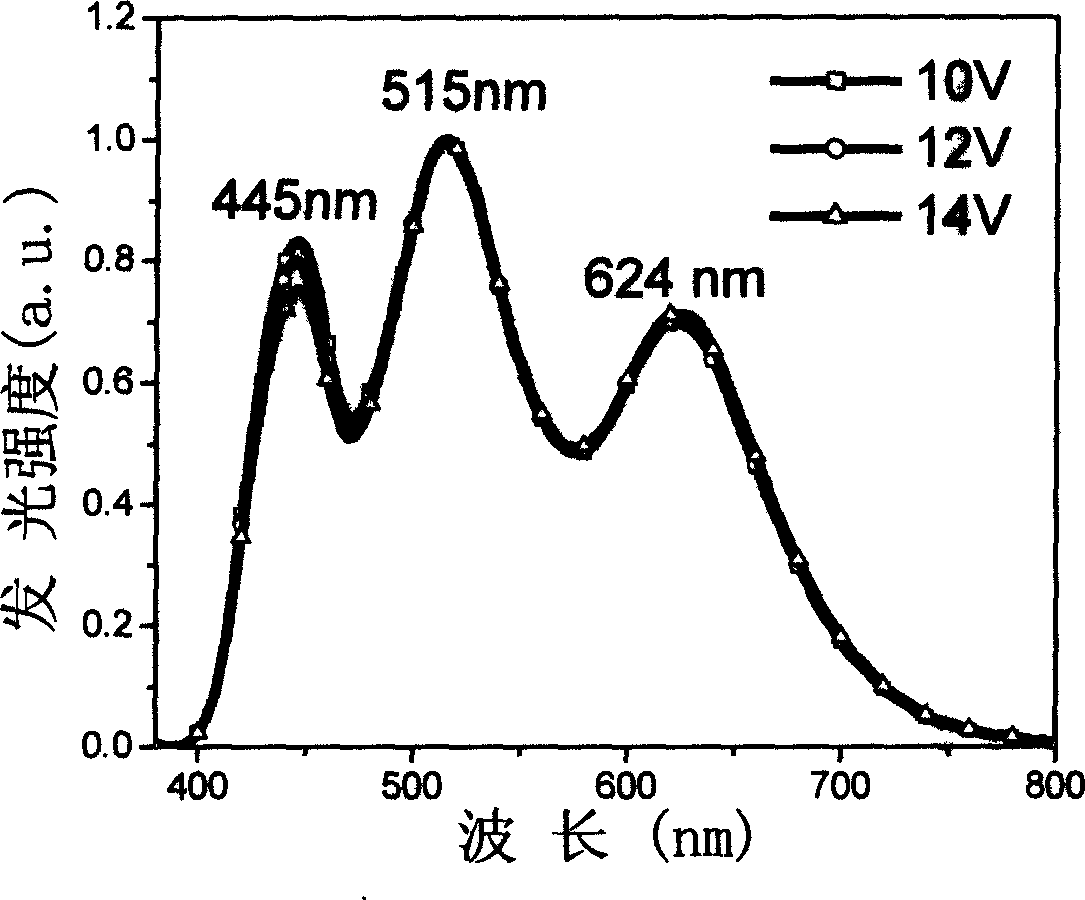
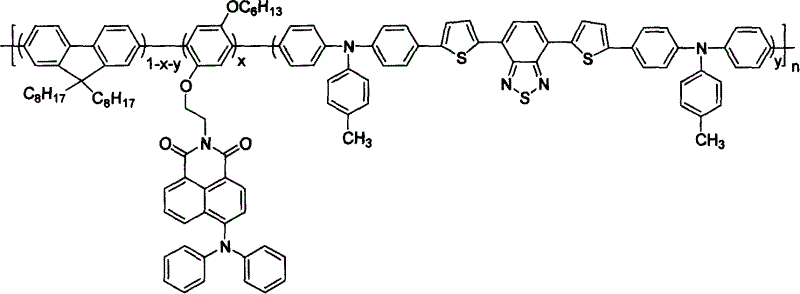
Three-color white light macromolecule luminescent material and method for preparing same
A technology of luminescent materials and polymers, applied in luminescent materials, chemical instruments and methods, sustainable buildings, etc., can solve the problems of background light sources that are not suitable for full-color display, and do not cover the visible light range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: 1,4-dibromo-2-hexyloxy-5-(2-(4-amino-1,8-naphthalimide-9-)ethoxy)benzene
[0027] Under the protection of nitrogen atmosphere, 4-amino-1,8-naphthalimide 5mmol (1.06g) was dissolved in dimethyl sulfoxide (60ml), then potassium hydroxide 6mmol (0.34g) was added to the solution, and electromagnetic stirring React at 120°C for ten minutes, then gradually add 5 mmol (2.31 g) of 2-hexyloxy-5-(2-bromoethoxy)-1,4-dibromobenzene to the system, react for 1 hour, and extract with chloroform After repeated washing, drying, filtration, and column chromatography to separate the product, the pure intermediate product 1,4-dibromo-2-hexyloxy-5-(2-(4-amino-1,8-naphthalimide-9 -) Ethoxy)benzene 2.06g, yield 70%.
Embodiment 2
[0028] Example 2: 1,4-dibromo-2-hexyloxy-5-(2-(4-diphenylamino-1,8-naphthalimide-9-)ethoxy)benzene
[0029] 1,4-dibromo-2-hexyloxy-5-(2-(4-amino-1,8-naphthalimide-9-)ethoxy)benzene 1.0mmol (0.589g), iodobenzene 2.0mmol (0.41g), potassium carbonate 2.0mmol (0.28g), 0.01mmol 18-crown-6 (0.003g), cuprous iodide 0.01mmol (0.002g), 1,3-dimethyl-3,4 , 5,6-tetrahydro-2-pyrimidinone (0.30 mL) was heated to 180° C. for 5 hours under nitrogen protection. After extraction with chloroform, it was washed repeatedly, dried, filtered, and the product was separated by column chromatography to obtain the pure intermediate product 1,4-dibromo-2-hexyloxy-5-(2-(4-diphenylamino-1,8-naphthalene Imide-9-)ethoxy)benzene 0.33 g, yield 45%.
Embodiment 3
[0030] Example 3: 2-(4-(N-phenyl-N-(4-methylphenyl)amino)phenyl)thiophene
[0031]2-Bromothiophene 20mmol (3.26g) ether solution (100ml) was slowly added dropwise to magnesium chips 30mmol (0.72g), then refluxed for 3 hours to obtain the ether solution of the Grignard reagent of 2-bromothiophene, and then Add 1-bromo-4-(N-phenyl-N-(4-methylphenyl)-amino)benzene 10mmol (3.38g) and 1,3-bis(diphenylphosphino)propane to this solution Combine nickel dichloride 0.1mmol (0.054g), reflux reaction for 24 hours, then pour the reaction system into dilute hydrochloric acid, wash with water several times, dry, concentrate, column separation, obtain the pure intermediate product 2-(4-(N- Phenyl-N-(4-methylphenyl)amino)phenyl)thiophene 1.50 g, yield 44%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com