Application of C21 steroid glycoside in pharmacy
A technology of glycosides and steroids, applied in the field of C21 steroid glycosides, can solve problems such as large side effects, application limitations, toxic side effects, etc., and achieve the effects of less toxic side effects, low price, and antidepressant effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
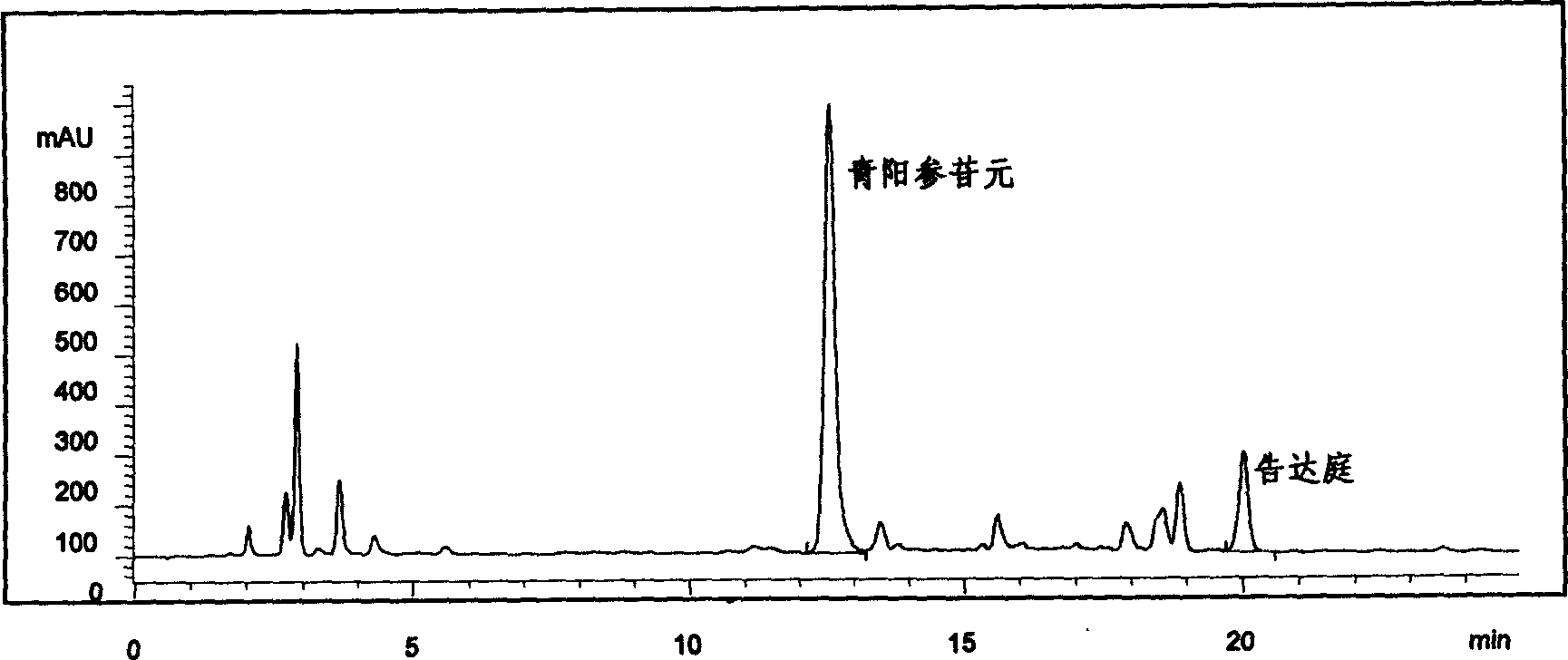
[0036] Take 10kg of the rhizome of Qingyangshen, crush it, extract it three times with 80% ethanol, combine the extracts, concentrate and recover the solvent. The concentrated solution was diluted with water, extracted three times with petroleum ether, the aqueous layer was extracted three times with chloroform, and the chloroform fractions were combined. Chloroform was recovered to obtain 300 g of light yellow powder, with a yield of 3.0%. Gained light yellow powder is the total glycosides of Qingyangshen, taking Qingyangshen aglycon as reference substance, referring to the drug standard (WS 3 -B-2343-97) method, the measured total glycoside content is 72.5%. The content of main aglycones in total glycosides was determined by HPLC method, and the results were: Qingyangshen aglycone content was 5.1%, and Kodatin content was 10.5%.
Embodiment 2
[0038] Take 10 kg of rhizomes of Kunming vine crown, crush them, extract three times with 80% ethanol, combine the extracts, concentrate and recover the solvent. The concentrated solution was diluted with water, extracted three times with petroleum ether, the aqueous layer was extracted three times with chloroform, and the chloroform fractions were combined. Chloroform was recovered to obtain 310 g of light yellow powder, with a yield of 3.1%. The obtained light yellow powder is the total glucosides of Kunming cuprosides. With Qingyang ginseng aglycone as the reference substance, refer to the drug standard (WS 3 -B-2343-97) method, the measured total glycoside content is 80.4%. The content of main aglycones in the total glycosides was determined by HPLC, and the results were: the content of Qingyangshen aglycone in the total glycosides was 6.0%, and the content of kudatingin was 15.2%.
Embodiment 3
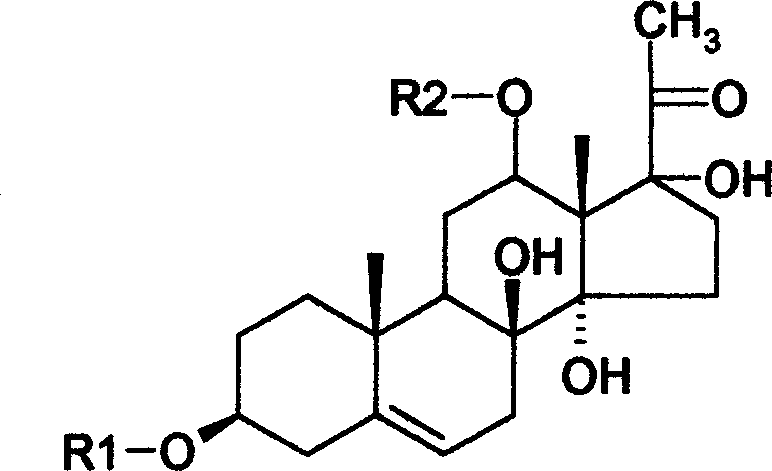
[0040]Take 50g of total glycosides of Qingyangshen, dissolve it in 200ml of methanol, add 200g of silica gel with a particle size of 100 mesh, stir evenly, and evaporate the methanol to dryness. Take 1000 g of silica gel with a particle size of 300-400 mesh, and perform column chromatography according to a conventional method, and the elution system is chloroform-methanol (20:1-5:1), gradient elution. The eluate was collected to obtain 5 fractions, and the third fraction was subjected to repeated column chromatography on reversed-phase silica gel, eluting with methanol: water (60:40), to obtain 1.2 g of compound 1, which was qingyang shenside A. White powder, molecular formula: C 56 h 86 o 16 , Molecular weight: 932. Soluble in ethanol, methanol, ethyl acetate, chloroform.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com