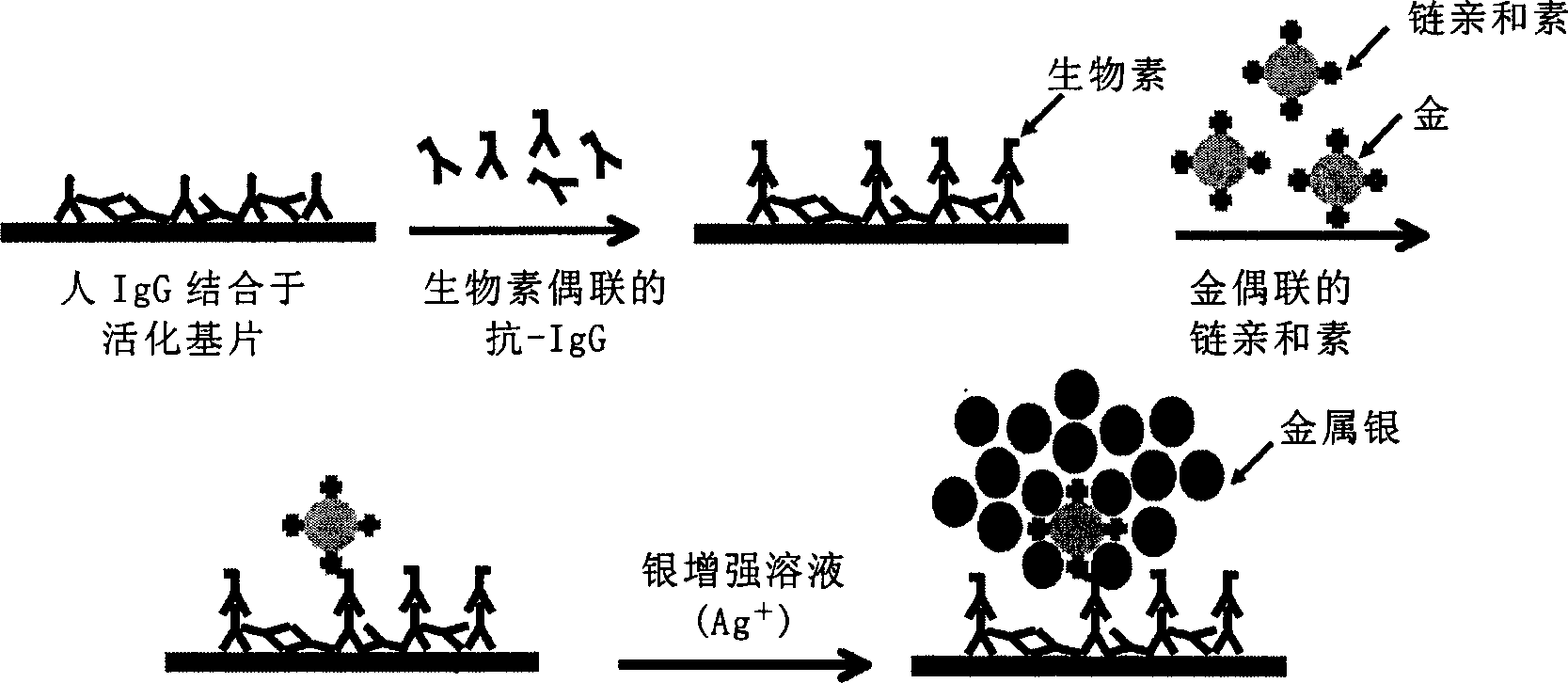
Protein chip detection by colloidal gold and silver reinforcing process
A protein chip, protein technology, applied in the biological field, can solve problems such as inapplicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] General detection conditions for colloidal gold-silver enhanced method
[0054] (a) The protein chip and the sample to be detected were reacted at room temperature for 1 hour, then washed with PBST (PBS containing 0.1% Tween 100), PBS and deionized water successively, and dried by centrifugation at 200g; first complex;
[0055] (b) reacting the protein chip in step (a) with the recognition molecule that recognizes the first complex to form a second complex composed of the first complex and the recognition molecule, wherein the recognition molecule has the first coupling The counterpart; the reaction conditions and washing and drying methods are the same as step (a);
[0056] (c) mixing the protein chip of step (b) with the colloidal gold solution with the second coupling partner to form a third complex composed of the second complex and colloidal gold, wherein the second coupling partner and Coupling is formed between the first coupling partners; the reaction conditio...
Embodiment 2
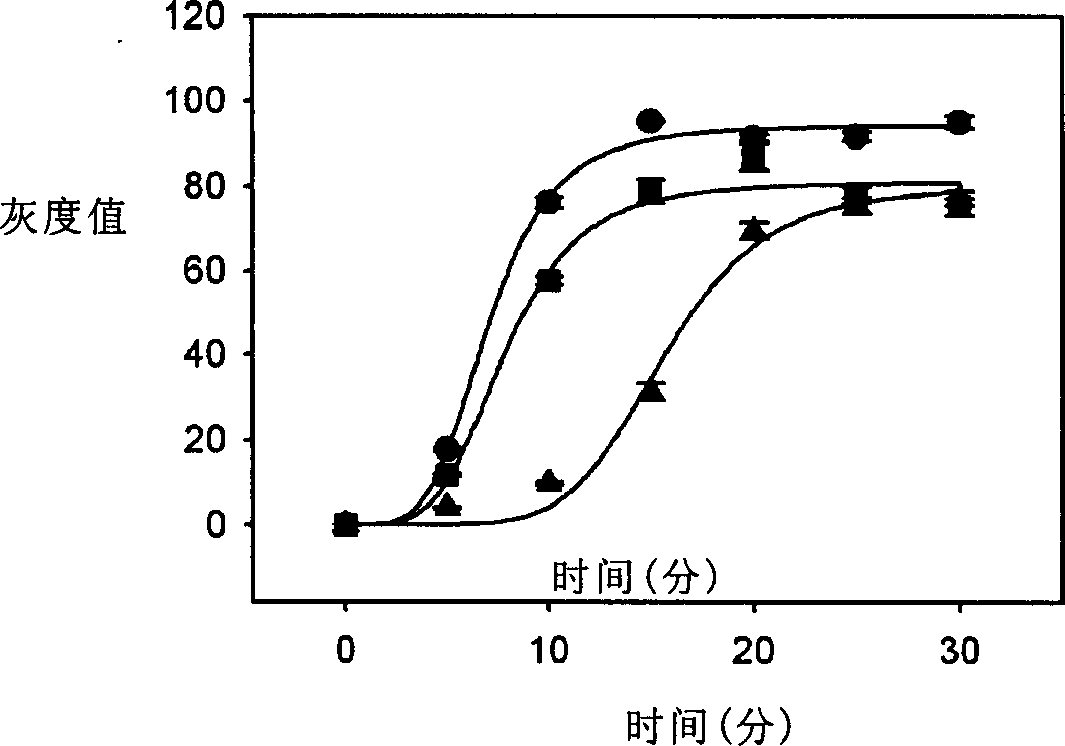
[0060] Influence of the mixing time of silver enhanced solution and protein chip on the results of detection by colloidal gold-silver enhanced method
[0061] The method of Example 1 was repeated, except that the silver enhancement solutions A and B were then mixed and reacted with the protein chip, and the reaction was stopped at 5, 10, 15, 20, 25 and 30 minutes, respectively. Then measure the gray value.
[0062] The result is as figure 2 As shown, at different concentrations (the spotting concentration of human IgG on the protein chip was 250 μg / ml. The concentrations of biotin-conjugated anti-human IgG were 110 ng / ml (▲), 1.1 μg / ml (■), and 11 μg / ml(●)), when the silver enhancement solution is mixed with the protein chip, the mixing time should be 5-30 minutes, more preferably 5-25 minutes.
Embodiment 3
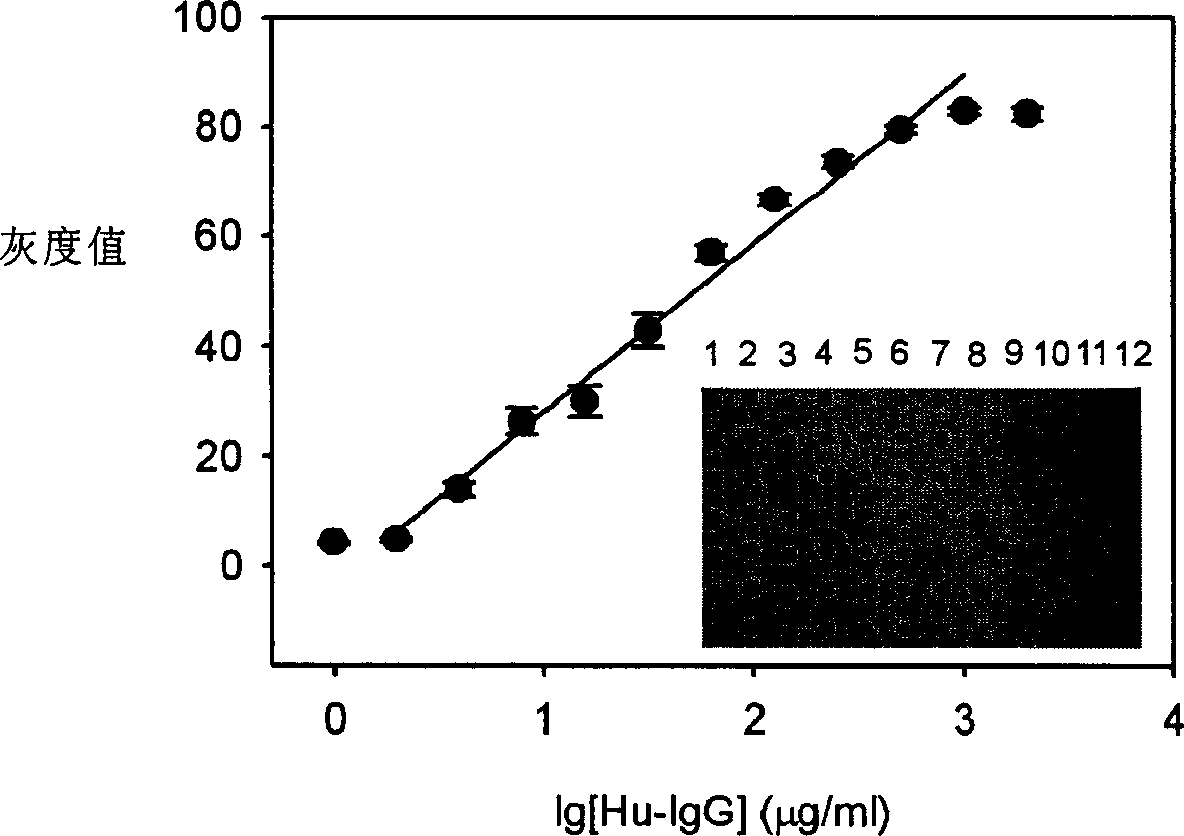
[0064] The effect of the number of spotting proteins bound to each spotting area in the protein chip on the results
[0065] The method of Example 1 was repeated, except that the quantity of the spotting protein bound to each spotting area in the protein chip was changed. Then measure the gray value.
[0066] The result is as image 3 As shown, the dependence of the gray value of the spots and the concentration of human IgG immobilized on the chip is shown. Wherein, the inner illustration is the colloidal gold-silver enhanced detection image of the microarray formed by different concentrations of human IgG. The spotting concentrations of human IgG in columns 1 to 12 are as follows; / ml, 7.8 μg / ml, 3.9 μg / ml, 1.95 μg / ml and 0.97 μg / ml. The concentration of biotin-conjugated anti-human IgG was 11 μg / ml; the silver enhancement time was 15 minutes. Data in the curves are taken as the mean of 7 replicate points in the inset.
[0067] The results showed that the lowest detecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com