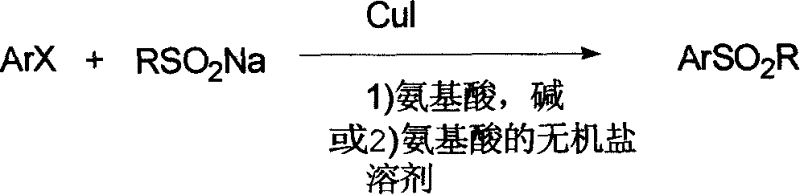
Amino acid accelerated CuI catalyzed aryl halide and coupling reaction of alkyl sulfonate
A technology of aryl halides and amino acids, which is applied in the field of coupling reaction between aryl halides and hydrocarbyl sulfinates, which can solve problems such as high temperature, environmental pollution, and limited substrate range, and achieve mild reaction conditions and low price , the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
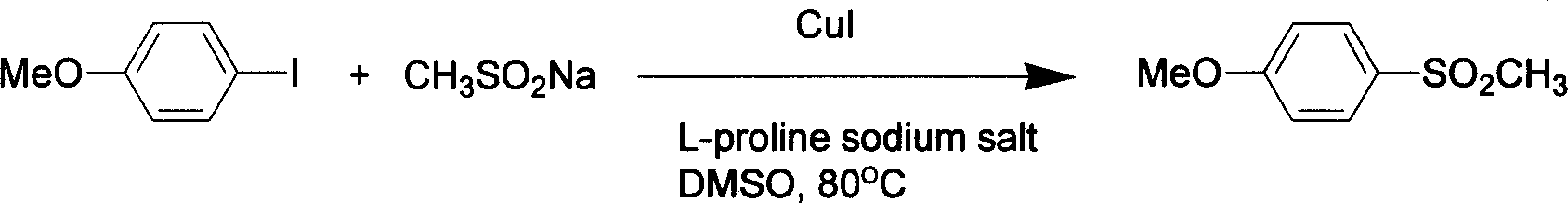
[0019] 1, Preparation of 4-methoxyphenylmethyl sulfone
[0020]
[0021] In a reaction tube, add 234mg p-methoxyiodobenzene (MW=234.04, 1.0mmol), then add 153mg methylsulfinic acid sodium salt (MW=102.09, 1.2mmol, content 80%), 28mg L-proline Sodium salt (MW=137.05, 0.2mmol), 19mg CuI (MW=190.45, 0.1mmol), 2ml DMSO as solvent, under the protection of nitrogen, react in 80 ℃ oil bath for 24 hours, cool, add 4 milliliters of water, every Once extracted with 10 ml of ethyl acetate, repeated three times, the extract was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was distilled under reduced pressure, and separated by a silica gel column (eluent petroleum ether: ethyl acetate=4: 1), to obtain 156 mg of product 4-methoxyphenylmethyl sulfone, yield 84%.
[0022] 1 H NMR (300MHz, CDCl 3 )δ 3.05(s, 3H), 3.90(s, 3H), 7.04(AB, J 1 =7.5Hz,J 2 =2.1Hz, 2H), 7.88 (AB, J 1 =7.5Hz,J 2 =2.1Hz, 2H); EI-MS (m / z) 186 (M + ), 171, 155, 139, 12...
Embodiment 2
[0025] 2, Preparation of 4-methylphenylmethyl sulfone
[0026]
[0027] In a reaction tube, add 218mg p-methyliodobenzene (MW=218.03, 1.0mmol), then add 153mg methylsulfinic acid sodium salt (MW=102.09, 1.2mmol, content 80%), 28mg L-sodium proline Salt (MW=137.05, 0.2mmol), 19mg CuI (MW=190.45, 0.1mmol), 2ml DMSO as solvent, under nitrogen protection, reacted in 80 ℃ oil bath for 24 hours, cooled, added 4 milliliters of water, each time Extracted with 10 ml of ethyl acetate, repeated three times, the extract was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was distilled under reduced pressure, separated by a silica gel column (petroleum ether:ethyl acetate=4:1 ), to obtain 158mg product 4-methylphenylmethyl sulfone, yield 93%.
[0028] 1 H NMR (400MHz, CDCl 3 )δ2.46(s, 3H), 3.04(s, 3H), 7.36(d, J=8.2Hz, 2H), 7.82(d, J=8.2Hz, 2H); EI-MS (m / z) 170 (M + ), 155, 139, 121, 107, 91, 77, 65, 51, 39.
Embodiment 3
[0030] 3. Preparation of 4-hydroxyphenylmethyl sulfone
[0031]
[0032] In a reaction tube, add 220mg p-hydroxyiodobenzene (MW=220.00, 1.0mmol), then add 153mg methylsulfinic acid sodium salt (MW=102.09, 1.2mmol, content 80%), 28mg L-sodium proline Salt (MW=137.05, 0.2mmol), 19mg CuI (MW=190.45, 0.1mmol), 2ml DMSO as solvent, under nitrogen protection, reacted in 80 ℃ oil bath for 24 hours, cooled, added 4 milliliters of water, each time Extracted with 10 ml of ethyl acetate, repeated three times, the extract was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was distilled under reduced pressure, separated by a silica gel column (petroleum ether:ethyl acetate=3:1 ), to obtain 160mg of product 4-hydroxyphenylmethyl sulfone, yield 93%.
[0033] 1 H NMR (400MHz, CDCl 3 )δ3.05 (s, 3H), 6.96 (d, J=8.7Hz, 2H), 7.74 (d, J=8.7Hz, 2H); EI-MS (m / z) 172 (M+ ), 157, 141, 109, 94, 79, 65, 43.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com