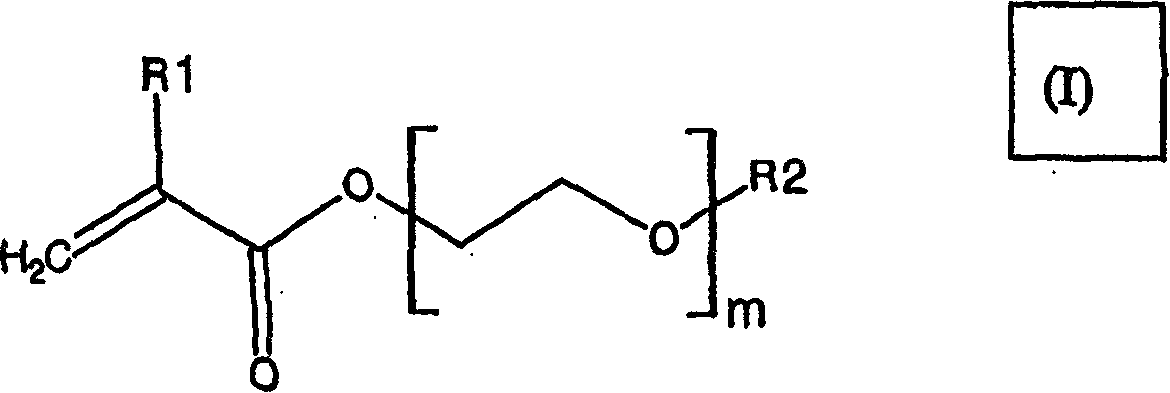
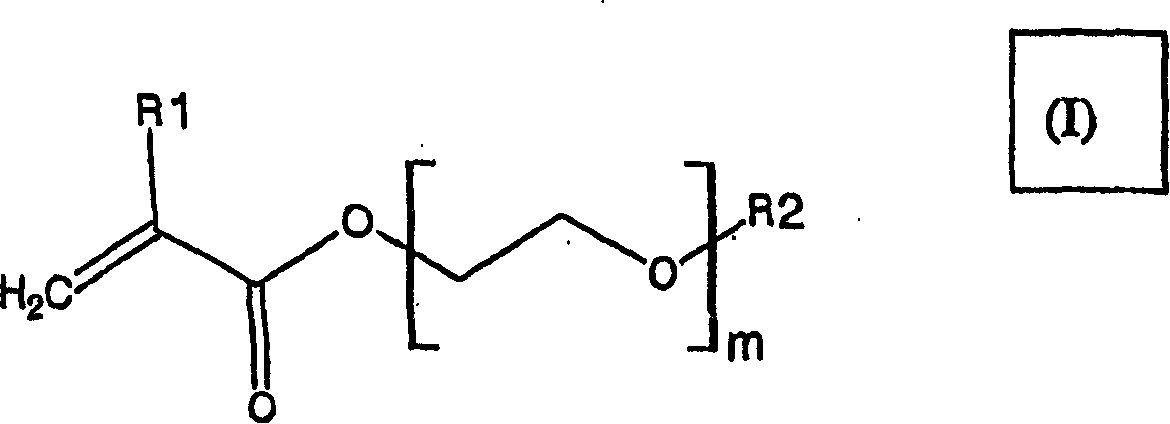
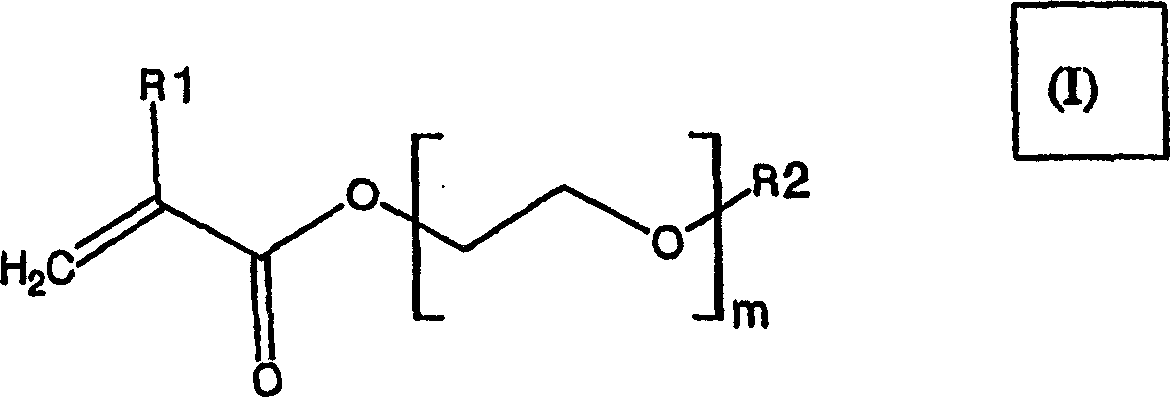
New film coating
A film and weight technology, applied in the direction of drug delivery, pharmaceutical formulations, etc., can solve the problems of film coating performance change, inappropriateness, stability impact, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Example 1: Synthesis of polymer dispersions using M1, M2 and M3
[0175] The polymerization was carried out using ethyl acrylate, 2-methyl methacrylate and monomers M1, M2 and M3.
[0176] Dispersions D1, D2 and D3 were prepared using the following components:
[0177] D1
[0178] Water 677.55g
[0179]Ethyl acrylate 217.75g
[0180] Methyl methacrylate 108.88g
[0181] Monomer M1 2.18g
[0182] Sodium dodecyl sulfate (SDS) 2.18g
[0183] NaHCO 3 (0.005M) 0.27g
[0184] Na 2 S 2 o 8 (Initiator) 1.67g
[0185] D2
[0186] Water 677.42g
[0187] Ethyl acrylate 218.23g
[0188] Methyl methacrylate 109.12g
[0189] Monomer M2 3.75g
[0190] SDS 2.19g
[0191] NaHCO 3 (0.005M) 0.27g
[0192] Na 2 S 2 o 8 1.72g
[0193] D3
[0194] Water 675.31g
[0195] Ethyl acrylate 218.85g
[0196] Methyl methacrylate 109.43g
[0197] Monomer M3 7.15g
[0198] SDS 2.19g
[0199] NaHCO 3 (0.005M) 0.27g
[0200] Na 2 S 2 o 8...
Embodiment 2
[0202] Example 2: Synthesis of polymer dispersion using M4
[0203] Dispersion D4 was prepared using the following components:
[0204] water 600g
[0205] Ethyl acrylate 120g
[0206] Methyl methacrylate 70g
[0207] Monomer M4 (hydroxyethyl methacrylate, see Table 1) 10g
[0208] SDS 3g
[0209] NaOH(1M) 2.3g
[0210] K 2 S 2 o 8 (Initiator) 0.6g
[0211] The monomer is distilled to remove the initiator. The emulsion polymerization was performed in a tightly capped water-jacketed vessel equipped with nitrogen sparging and stirring. Disperse monomer, SDS and sodium hydroxide in water and stir (50 rpm). The temperature was raised to 50°C and the initiator was added. After the polymerization was carried out for 20 hours, the temperature was set to 70°C and carried out for another 2 hours. The dispersion was then filtered and cooled.
Embodiment 3
[0212] Embodiment 3: prepare film F1-F4 by embodiment 1 and 2
[0213] About 10 ml each of the dispersions D1-D4 were poured into a Teflon mold to obtain monomer films F1-F4. The mold was then placed in a temperature and humidity chamber at 25° C. and 60% relative humidity for 19 hours for drying and film formation.
[0214] result:
[0215] The tackiness of the film was tested by simply handling the film manually. The films were subjected to permeability testing as described in Example 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com