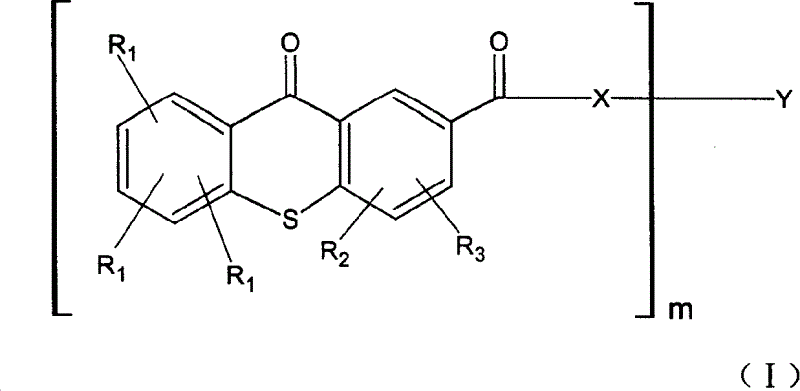
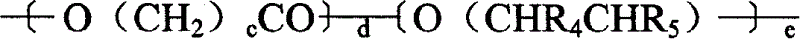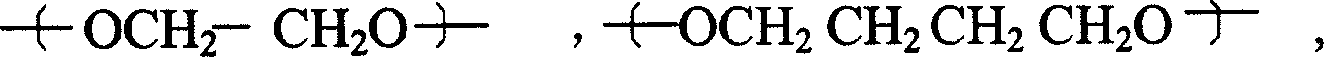Light mitiator of thioxanthone-2-carboxylic ester
A technology of C1-C12 compounds, applied in the field of novel thioxanthone carboxylate and diester derivatives, can solve problems such as danger and violent reaction, and achieve the effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: the preparation of ethyl thioxanthone-2-carboxylate:
[0067] In a 10L four-necked flask equipped with mechanical stirring, add 445g of thioxanthone-2-carboxylic acid and 2500ml of thionyl chloride, heat to reflux, a large amount of hydrogen chloride gas is generated, and continue to reflux for 5 hours. Heating was stopped, and excess thionyl chloride was distilled off. Add 2.5L of anhydrous toluene to dissolve the residue, add 1500ml of absolute ethanol at room temperature, raise the temperature, reflux for 3-4 hours, distill off the ethanol, quickly add 1000ml of saturated potassium carbonate solution under stirring, separate the water phase, and toluene The phase was filtered, and the filtrate was cooled slowly. 325 g of pale yellow needle-like crystals were obtained, and the toluene solution was concentrated for further crystallization to obtain 155 g. The yield was 91%, and the product purity (HPLC) was 98.67%.
Embodiment 2
[0068] Embodiment 2: Thioxanthone-2-carboxylic acid polytetrahydrofuran-250-diester:
[0069] 28.4 grams (0.1ml) of ethyl thioxanthone-2-carboxylate, 12.5 grams (ml) of polytetrahydrofuran (average molecular weight 250) and 0.5 gram of butyl stannic acid, under nitrogen protection, heated at 170°C for 8 hours, cooled at 150°C to reduce Remove ethanol by pressure for about half an hour, after cooling, add 200ml tetrahydrofuran, heat and reflux for one hour, then add a little activated carbon, cool to room temperature, filter to remove catalyst, mother liquor is firstly removed tetrahydrofuran by rotary evaporation, and then dried at 80°C / 1mbar, leaving 36.18 grams of oily matter, productive rate 97%, HPLC proves product purity,
[0070] Elemental analysis: sulfur: calculated value: 8.82% test value: 8.61%
Embodiment 3
[0071] Example 3: Thioxanthone-2-carboxylic acid polyethylene glycol-400-diester:
[0072] The steps of Example 2 were repeated, except that polyethylene glycol-400 was used instead of polytetrahydrofuran-250 to obtain thioxanthone-2-carboxylic acid polyethylene glycol-400-diester.
[0073] Elemental analysis: sulfur: calculated value: 7.23% test value: 7.45%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com