Itraconazole hydrochloride, oral solid combination and preparation method
A technology of itraconazole hydrochloride and solid composition, which is applied in the field of oral solid composition and preparation containing itraconazole hydrochloride, can solve the problems of low heating temperature and production without industrialized setting equipment, and achieve Low energy consumption, conducive to environmental protection and large-scale industrial production, and the effect of water solubility improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of itraconazole hydrochloride
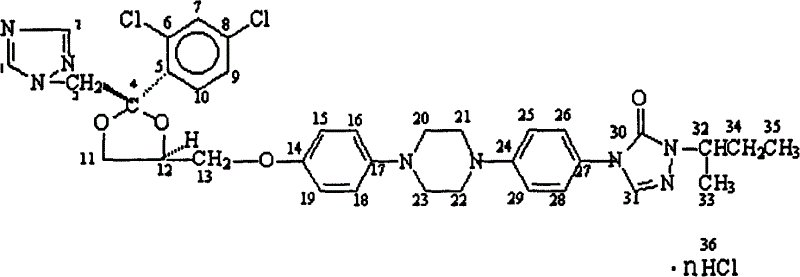
[0042] Add 40 g of itraconazole and 600 ml of acetone into a 1 L reaction flask, and feed excess hydrogen chloride under reflux and stirring. After the reaction is complete, filter, wash with acetone, and dry to obtain 42 g of itraconazole hydrochloride. Yield 95%. Elemental Analysis C 35 h 38 N 8 o 4 .2 HCl: (experimental / calculated) C53.70 / 53.99, H5.20 / 5.18, N14.46 / 14.39, Cl18.20 / 18.22.
[0043] Itraconazole hydrochloride 1 HNMR data (using deuterated dimethyl sulfoxide as solvent)
[0044]
[0045] proton number
Embodiment 2
[0047] Preparation of itraconazole hydrochloride
[0048] Add 2g of itraconazole and 20ml of ethanol into a 50ml reaction bottle, heat to reflux, pass in excess hydrogen chloride under stirring, after the reaction is complete, cool to room temperature, filter, wash with ethanol, and dry to obtain itraconazole Hydrochloride 2.12g. Yield 96.4%. elemental analysis and 1 HNMR data same as above.
Embodiment 3
[0050] Preparation of capsules containing itraconazole hydrochloride (100 mg itraconazole / capsule)
[0051] 1. Prescription
[0052] Itraconazole Hydrochloride 110g
[0053] β-cyclodextrin 250g
[0054] Makes 1000 capsules
[0055] 2. Craft
[0056] 2.1 Pass itraconazole hydrochloride through a 200-mesh sieve;
[0057] 2.2 Weigh 110 grams of itraconazole hydrochloride and 250 grams of β-cyclodextrin, mix well, and pass through a 60-mesh sieve three times.
[0058] 2.3 Fill the mixed powder of 2.2 into the No. 0 capsule.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com