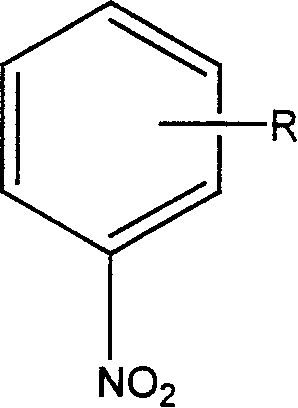
Degradation of nitrobenzol fomite with catalytic wetting shared oxidative method
A technology of nitrobenzene and co-oxidation, which is applied in the direction of protection devices against harmful chemicals, can solve the problems of high toxicity, mutagenesis, teratogenicity, etc., and achieves high treatment concentration, reduced energy consumption, and increased activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1. Wet oxidation method directly treats nitrobenzene waste water
[0022] Put 200ml of nitrobenzene wastewater solution with a concentration of 100mg / L to 400mg / L into the reactor, and replace it with ultra-pure nitrogen for 3 times (the purpose of replacing with nitrogen is to calculate the reaction time and determine the removal rate benchmark), and then add to the system Fill the medium with ultra-pure nitrogen at a certain pressure, heat up to the specified temperature, then fill with high-purity oxygen of 0.2-5Mpa, stir the reaction solution at a speed of 500 rpm, and react for 1 hour. After the reaction, the nitrobenzene compound The removal rate is shown in Table 1.
[0023] Reactant
[0024] Due to the difference in the substitution position of the hydrocarbon group, the removal rate will be different, mainly due to the influence of steric hindrance.
Embodiment 2
[0025] Embodiment 2, catalytic wet co-oxidation process nitrobenzene waste water
[0026] The difference from Example 1 is that methanol, ethanol, isopropanol, phenol and aniline are added respectively to the nitrobenzene waste water solution, and a catalyst is added simultaneously (the specific consumption is 0.2-5.0g), and after the reaction, the nitrobenzene The removal rate of similar wastewater is shown in Table 2.
[0027] Reactant
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com