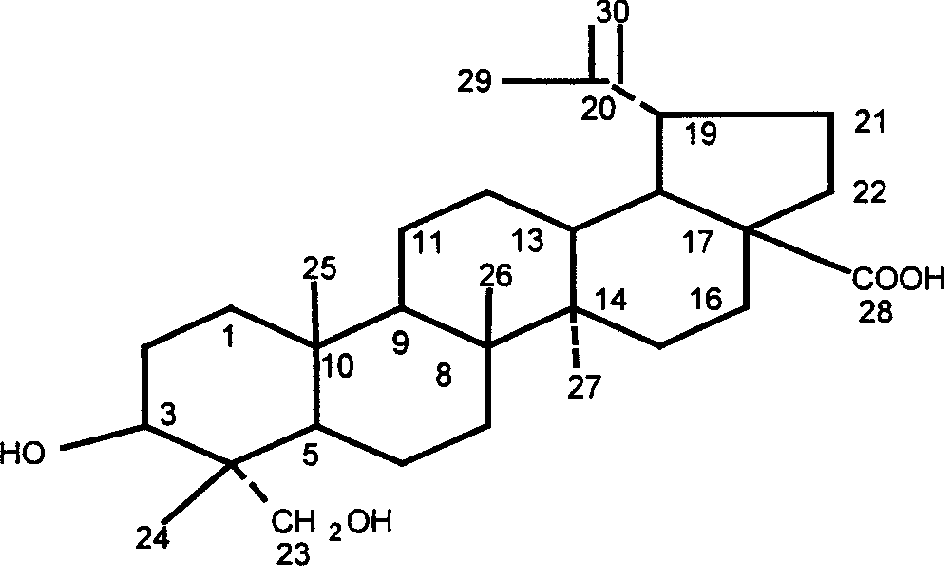
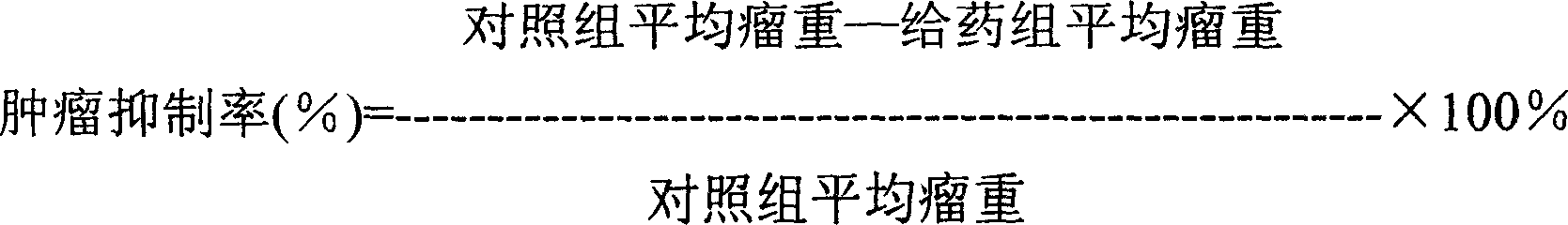
Anti-cancer medicine 23-hydroxy betulic acid fat emulsion and its preparing method
An anti-tumor drug, fat emulsion technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as the inability to effectively solve bioavailability, and achieve the advantages of increasing clinical application dosage forms, improving bioavailability, and significant effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 23-Hydroxybetulinic acid fat emulsion, which consists of:
[0057] 23-Hydroxybetulinic acid 0.5g
[0058] Soy Lecithin for Injection 5g
[0059] Soybean oil for injection 100g
[0060] Glycerin 18g
[0061] Add water for injection to 1000mL
[0062] The preparation method of above-mentioned fat emulsion:
[0063] 1. Grinding 23-hydroxy betulinic acid so that the particle size of 23-hydroxy betulinic acid is less than 75 μm;
[0064] 2. Weigh the crushed 23-hydroxybetulinic acid of the prescribed amount, add the prescribed amount of soybean oil for injection, and mix well to form the oil phase;
[0065] 3. Weigh soybean lecithin for injection, glycerin and appropriate amount of water for injection (at least to completely dissolve the phospholipid into the water phase) of the prescribed amount, and stir at high speed (8000-20000 rpm) after mixing to completely dissolve into the water phase;
[0066] 4. Under the condition of high-speed stirring, add the oil phase in...
Embodiment 2、3、4
[0072] The processing step of embodiment 2,3,4 is exactly the same as embodiment 1, and each component and consumption are as shown in table 1 in its prescription:
[0073] Example
Embodiment 5、6、7、8、9、10
[0075] The processing step of embodiment 5,6,7,8,9,10 is exactly the same as embodiment 1, and each component and consumption are as shown in table 2 in its prescription:
[0076]
Example
(0.5-5g)
(100-250g)
Emulsifier
(5-20g)
osmotic regulator
(18-25g)
5
23-Hydroxybetulinic acid
glucose
6
23-Hydroxybetulinic acid
7
23-Hydroxybetulinic acid
8
23-Hydroxybetulinic acid
glucose
9
23-Hydroxybetulinic acid
saffron oil
10
23-Hydroxybetulinic acid
fatty acid triglycerides
[0077] In the foregoing examples, the vegetable oil soybean oil used for injection can also be replaced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com