Preparation method and application of paclitaxel intravenous fat emulsion
A paclitaxel and fat emulsion technology, applied in the field of medicine, can solve the problems of toxic reaction, influence on the clinical safe application of taxane drugs, anaphylactic shock, etc., and achieve the effects of enhanced compliance, simple preparation method, and uniform quality and appearance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of paclitaxel fat emulsion with seal oil as oil phase and polyoxyethylene 40 hydrogenated castor oil as emulsifier
[0049] Accurately weigh 0.1g paclitaxel and dissolve in 1.0g seal oil as the oil phase; weigh 0.25g polyoxyethylene 40 hydrogenated castor oil (HCO-40) and 0.20g glycerin in 10ml water for injection (0.1% sodium hydroxide to adjust pH Mix evenly in 8.5) and use it as the water phase; slowly add the oil phase to the water phase dropwise, and stir for 30 minutes with magnetic force to form a primary emulsion. fat milk.
Embodiment 2
[0050] Example 2: Preparation of paclitaxel fat emulsion with seal oil as oil phase and lecithin as emulsifier
[0051] Accurately weigh 0.02g of paclitaxel and dissolve in 1.0g of seal oil as the oil phase; weigh 0.25g of lecithin and 0.225g of glycerin in 10ml of water for injection (0.1% sodium hydroxide to adjust the pH to 8.5) and mix evenly as the water phase Then slowly drop the oil phase into the water phase, stir it magnetically for 30 minutes to form a primary emulsion, and circulate the high-pressure emulsion 5 times under a pressure of 800-1200 bar to obtain paclitaxel intravenous fat emulsion.
Embodiment 3
[0052] Example 3: Observation of Microscopic Morphology of Paclitaxel Fat Emulsion
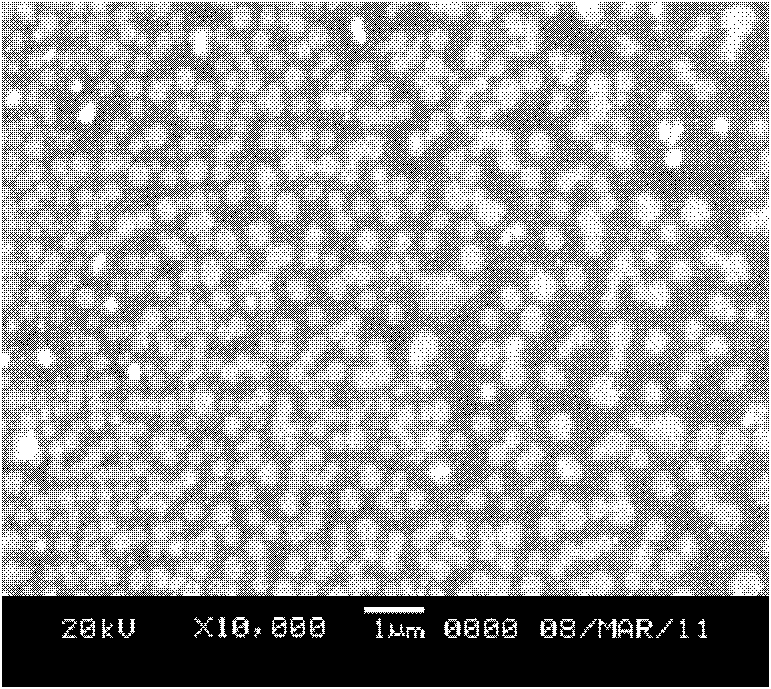
[0053] Take the paclitaxel fat emulsion sample solution, dilute it with distilled water, take 10ul and put it on the surface of the copper mesh, line it with filter paper, and dry it naturally, then prepare for transmission electron microscope observation. Fix the electron beam voltage of the electron microscope at 80kV, move the coordinates, adjust the magnification, find and observe the particles and take pictures. See attached figure 1 . At the same time, 20ul of the diluted sample was dropped on the cover glass, evaporated to dryness naturally, sprayed with gold, and observed by scanning electron microscope. See attached figure 2 .
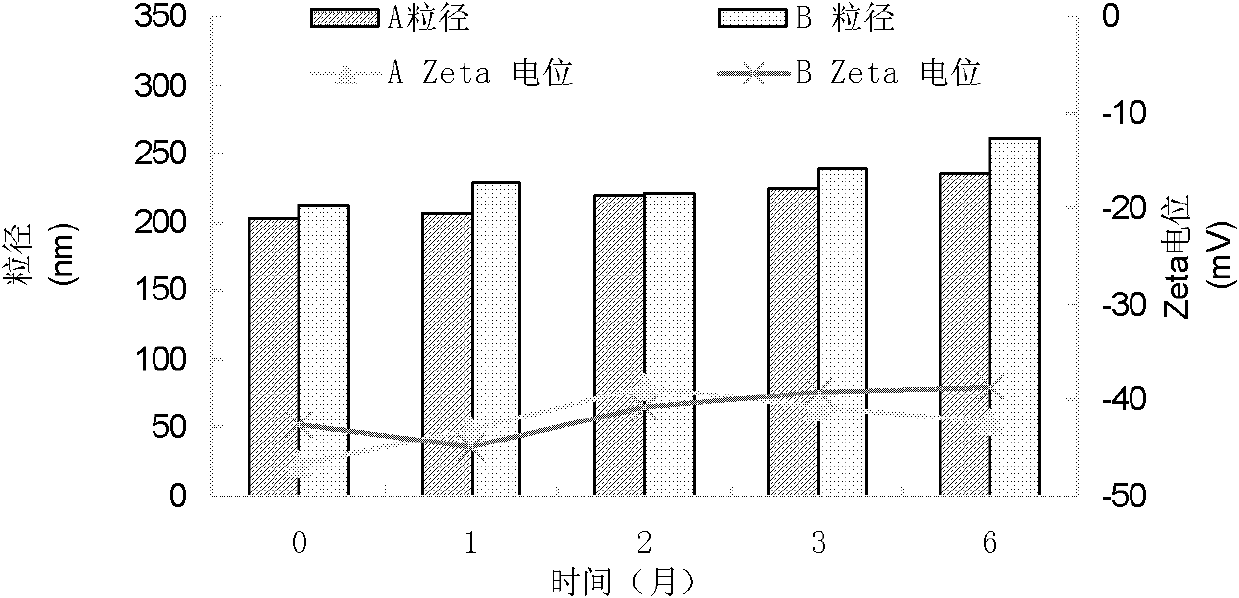
[0054] In the present invention, it can be seen through a transmission electron microscope that a single nanostructured lipid carrier is a spherical particle with relatively uniform size and relatively uniform distribution; the above results are also con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com