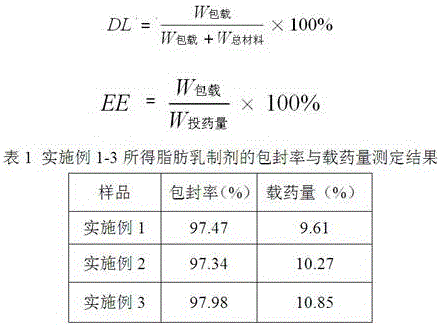
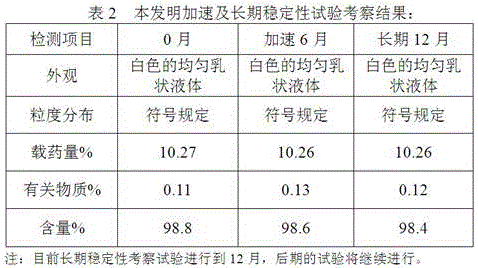
A kind of posaconazole fat emulsion injection and preparation method thereof
A technology for posaconazole fat and posaconazole, which is applied in the directions of emulsion delivery, oil/fat/wax inactive ingredients, pharmaceutical formulations, etc., can solve problems such as low solubility, achieve simple preparation process and improve stability , good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
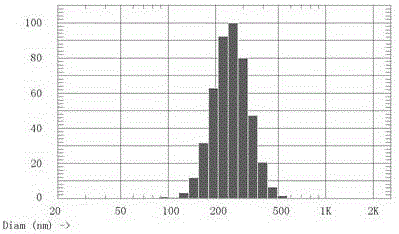
Image
Examples
Embodiment 1
[0031] Embodiment 1: 1000ml posaconazole fat emulsion injection preparation method
[0032] Under the protection of nitrogen, mix 100 g of soybean oil and 10 g of soybean lecithin evenly, heat to 80 ° C, add 18 g of the main drug posaconazole while stirring, and mix well to obtain an oil phase;
[0033] Add 50g of mannitol to 750ml of water for injection, dissolve evenly, and heat to 80°C at the same time to obtain the water phase;
[0034] Under the protection of nitrogen, the obtained oil phase was added dropwise to the water phase under high-speed stirring (2500rpm), kept stirring for 5 minutes, adjusted the pH to 2.0 with a pH regulator, added water for injection to the full amount, and mixed well to obtain the initial Emulsion;
[0035] Under the protection of nitrogen, at room temperature, the obtained primary emulsion was circulated in a high-pressure homogenizer for 4 times under high pressure (the set homogeneous pressure was 600bar) to obtain an emulsion;
...
Embodiment 2
[0037] Embodiment 2: Preparation method of 1000ml posaconazole fat emulsion injection
[0038] Under nitrogen protection, mix 100 g of soybean oil and 20 g of soybean lecithin evenly, heat to 75°C, add 20 g of the main drug posaconazole while stirring, and mix well to obtain an oil phase;
[0039] Add 50g of mannitol to 700ml of water for injection, dissolve evenly, and heat to 75°C at the same time to obtain the water phase;
[0040] Under the protection of nitrogen, the obtained oil phase was added dropwise to the water phase under high-speed stirring (1800rpm), kept stirring for 5 minutes, adjusted the pH to 2.6 with a pH regulator, added water for injection to the full amount, and mixed well to obtain the initial Emulsion;
[0041] Under the protection of nitrogen, at room temperature, the obtained primary emulsion was circulated in a high-pressure homogenizer for 4 times under high pressure (the set homogeneous pressure was 400bar) to obtain an emulsion;
[0042...
Embodiment 3
[0043] Embodiment 3: 1000ml posaconazole fat emulsion injection preparation method
[0044] Under nitrogen protection, mix 75g of soybean oil, 5g of soybean lecithin and 5g of poloxamer evenly, heat to 75°C, add 18g of the main drug posaconazole while stirring, and mix well to obtain an oil phase;
[0045] Add 55g of glucose to 650ml of water for injection, dissolve evenly, and heat to 75°C at the same time to obtain the aqueous phase;
[0046] Under the protection of nitrogen, the obtained oil phase was added dropwise to the water phase under high-speed stirring (2500rpm), kept stirring for 5 minutes, adjusted the pH to 3.0 with a pH regulator, added water for injection to the full amount, and mixed well to obtain the initial Emulsion;
[0047] Under the protection of nitrogen, at room temperature, the obtained primary emulsion was circulated in a high-pressure homogenizer for 4 times under high pressure (the set homogenization pressure was 500 bar) to obtain an emuls...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com