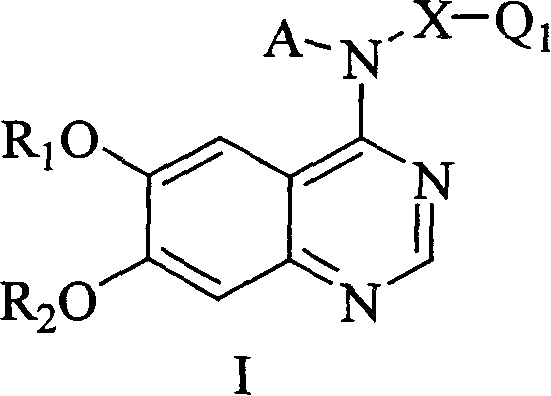
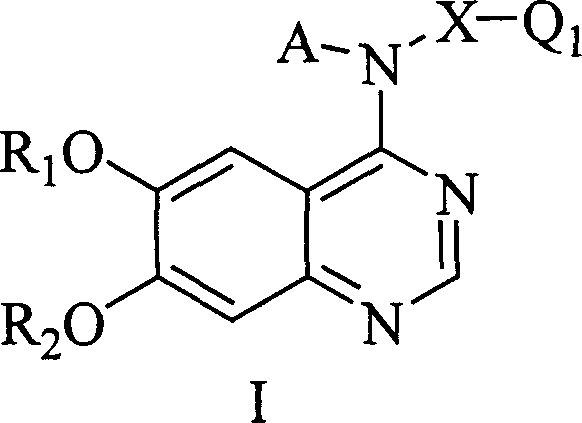
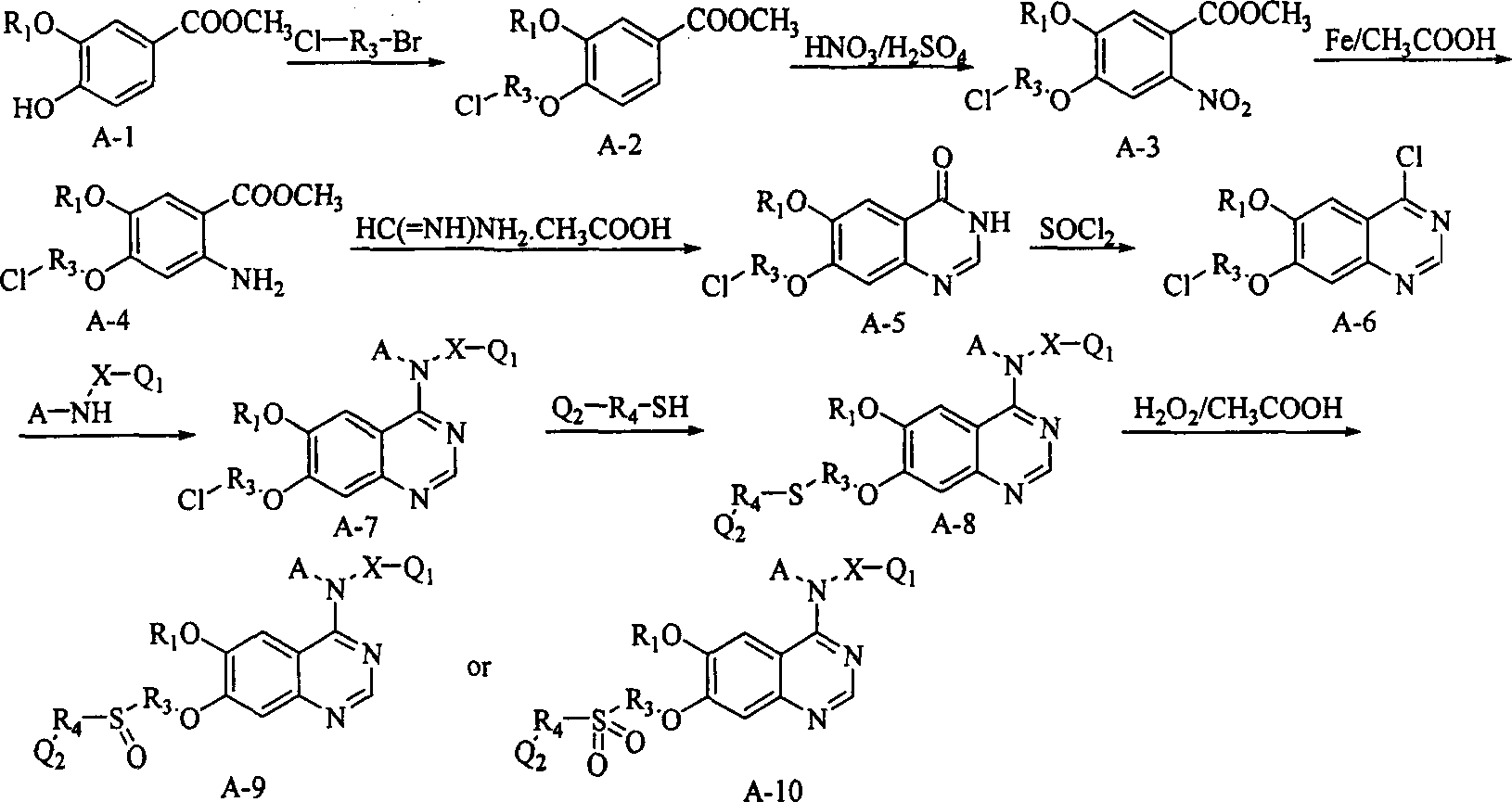
New quinazoline hind derivative, medicinal composition containing same and their use
A technology of derivatives and mixtures, applied in the field of 4-substituted anilinoquinazoline derivatives, can solve the problems of blocking cell differentiation regulation function, inducing tumors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Example 1: 4-(3-chloro-4-fluoroanilino)-6-methoxy-7-[[3-[(5-dimethylamino)methylfuran-2-]methylthio] Propoxy]quinazoline oxalate
[0156]Step A: Preparation of methyl 3-methoxy-4-(3-chloropropoxy)benzoate
[0157] In 150 mL of dimethylformamide (DMF), add 9.1 g (0.05 mol) of methyl 3-methoxy-4-hydroxybenzoate, 15.8 g (0.1 mol) of 1,3-bromochloropropane, anhydrous carbonic acid Potassium 10.4g (0.075mol), stirred at 70-80°C for 6h. After the reaction was completed, the reaction solution was poured into ice water, extracted with dichloromethane, the organic phases were combined, washed with water, dried over anhydrous magnesium sulfate, evaporated to remove the solvent, and dried to obtain 12.3 g of the product (yield: 95.3%).
[0158] Step B: Preparation of methyl 2-nitro-4-(3-chloropropoxy)-5-methoxybenzoate
[0159] 12.3 g (0.048 mol) of methyl 3-methoxy-4-(3-chloropropoxy)benzoate was added to 100 mL of chloroform, and stirred to dissolve. Cool to about 10°C, slow...
Embodiment 2-39
[0173] According to the method of Example 1, using methyl 3-methoxy-4-hydroxybenzoate and 1,3-bromochloropropane or 1,2-bromochloroethane as starting materials, first prepare 4-chloro- 6-methoxy-7-(3-chloroalkoxy) quinazoline, then with suitable amine substitution reaction, then with furanmethylthiol substitution reaction, and then carry out Mannich reaction with suitable aliphatic amine, respectively Compounds of Examples 2-39 were obtained:
Embodiment 2
[0174] Example 2: 4-(3-chloro-4-fluoroanilino)-6-methoxy-7-[[3-[5-(N-methylethylamino)methylfuran-2-]methanol Thio]propoxy]quinazoline oxalate
[0175] 1 H-NMR(DMSO): 1.20(t, 3H), 2.05(m, 2H), 2.62(s, 3H), 2.68(t, 2H), 2.99(q, 2H), 3.82(s, 2H), 3.96 (s, 3H), 4.19(t, 2H), 4.29(s, 2H), 6.39(d, 1H), 6.61(d, 1H), 7.20(s, 1H), 7.44(t, 1H), 7.81( m, 1H), 7.86(s, 1H), 8.13(dd, 1H), 8.51(s, 1H); MS: 545.2(M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com