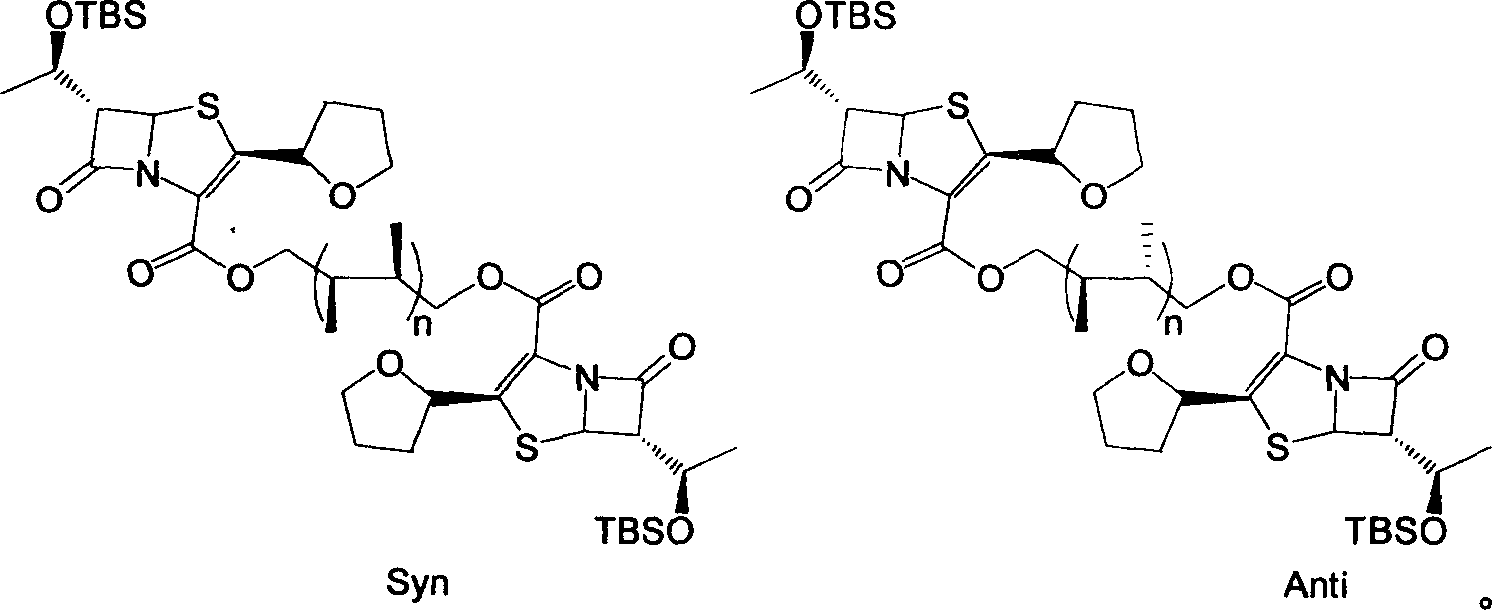
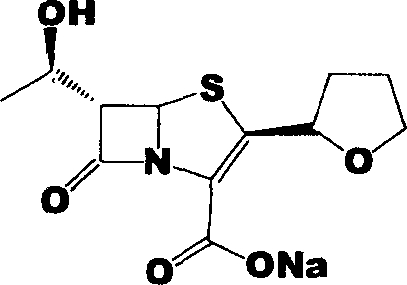
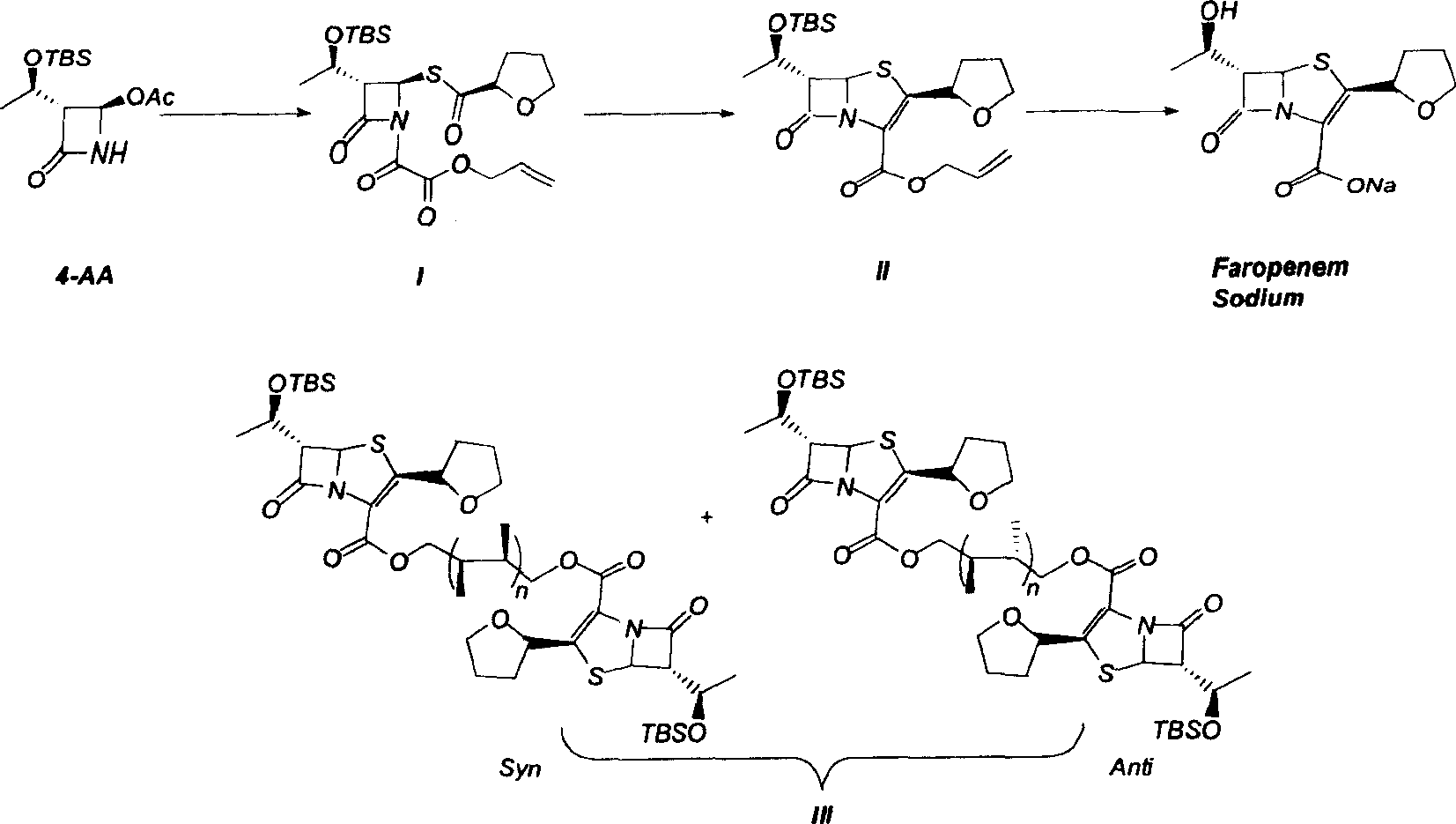
Faropenem sodium synthesis method from reaction by-product
A technology of faropenem sodium and by-products, which is applied in the field of synthesis of faropenem sodium from reaction by-products, can solve the problems of low yield and high cost of faropenem sodium, achieve strong controllability, easy operation, and increase the total yield of preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of Faropenem Sodium
[0022] Add 35g of compound III to the three-neck flask, dissolve it with 400mL THF, add 60mL of 15% hydrofluoric acid, raise the temperature to 65°C and react for about 18h, after TLC monitors the reaction is complete, add 200mL of water, extract with 400mL ethyl acetate, and Wash with deionized water, 100 mL saturated sodium bicarbonate solution, 100 mL saturated sodium bisulfate solution and 100 mL saturated brine, collect the organic phase, and dry over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to obtain the deprotected product (light yellow solid 20 g), which was dissolved in 150 mL of ether and transferred to a constant pressure funnel for the next step.
[0023] Add 12g of sodium 2-ethylhexanoate, 4g of triphenylphosphine, and 1.8g of tetrakis(triphenylphosphine)palladium to the above ether solution, and stir the reaction at 50°C. After 6 hours, a large amount of pale yellow solids are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com