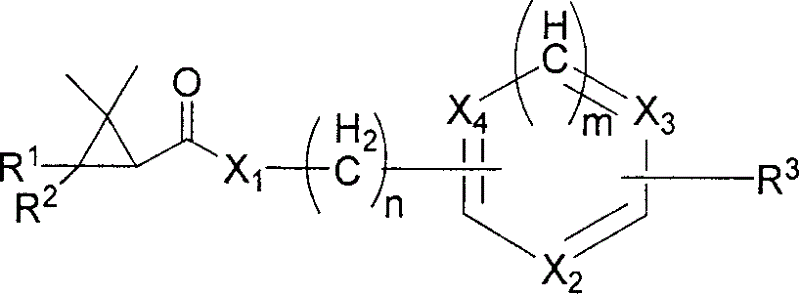
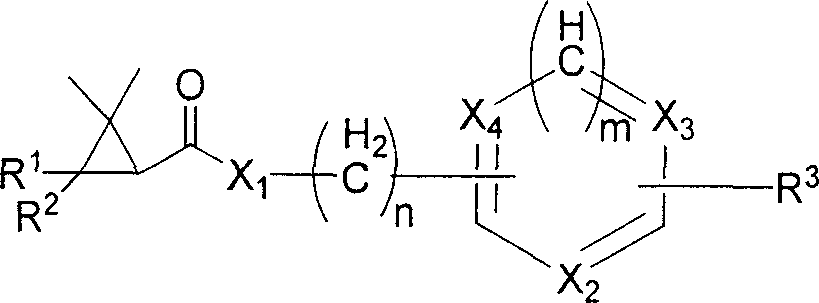Heterocyclicmethrin compound and its uses in weeding and sterilization
A technology of heterothrins and compounds, which is applied in the field of heterothrins and their application in herbicides and fungicides, and can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation and activity of 4-methyl-2-hydroxyl-3-nitropyridine trans-D-DV permethrin
[0022] Preparation: Prepare 4-methyl-2-hydroxy-3-nitropyridine and equimolar amounts of triethylamine into a dichloromethane solution with a concentration of 0.5mol / L. Take 2mL of this solution into the reaction flask, shake at room temperature for 0.5h, and cool the reaction system. Similarly, the trans-dextrorotatory DV chrysanthemum acid chloride was formulated into a 0.5 mol / L dichloromethane solution (DMC). After the reaction system was cooled to -5°C, it was maintained for 20 minutes, and 2 mL of the chrysanthemic acid chloride solution prepared earlier was added to the reaction flask in an equimolar amount. After 1 hour, the cycle freezing was stopped, and it was allowed to rise to room temperature naturally, while keeping shaking overnight. The progress of the reaction was detected by TLC. After the reaction was completed, 3 mL of saturated sodium bicarbonate solut...
Embodiment 2
[0024] Example 2 Preparation and activity of 2-amino-6-picoline trans-D-DV permethrin
[0025] Preparation: Prepare 2-amino-6-picoline and equimolar amounts of triethylamine into a dichloromethane solution with a concentration of 0.5mol / L. Take 2mL of this solution into the reaction flask, shake at room temperature for 0.5h, and cool the reaction system. Similarly, the trans-dextrorotatory DV chrysanthemum acid chloride was formulated into a 0.5 mol / L dichloromethane solution (DMC). After the reaction system was cooled to -5°C, it was maintained for 20 minutes, and 2 mL of the chrysanthemic acid chloride solution prepared earlier was added to the reaction flask in an equimolar amount. After 1 hour, the cycle freezing was stopped, and it was allowed to rise to room temperature naturally, while keeping shaking overnight. The progress of the reaction was detected by TLC. After the reaction was completed, 3 mL of saturated sodium bicarbonate solution was added to the reaction bot...
Embodiment 3
[0027] Example 3 Preparation and activity of 2-hydroxy-5-nitropyridine trans-D-DV permethrin
[0028] Preparation: Prepare 2-hydroxy-5-nitropyridine and equimolar amounts of triethylamine into a dichloromethane solution with a concentration of 0.5mol / L. Take 2mL of this solution into the reaction flask, shake at room temperature for 0.5h, and cool the reaction system. Similarly, the trans-dextrorotatory DV chrysanthemum acid chloride was formulated into a 0.5 mol / L dichloromethane solution (DMC). After the reaction system was cooled to -5°C, it was maintained for 20 minutes, and 2 mL of the chrysanthemic acid chloride solution prepared earlier was added to the reaction flask in an equimolar amount. After 1 hour, the cycle freezing was stopped, and it was allowed to rise to room temperature naturally, while keeping shaking overnight. The progress of the reaction was detected by TLC. After the reaction was completed, 3 mL of saturated sodium bicarbonate solution was added to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com