Preparation method of ertabeinan sodium salt
A technology of penem sodium and sodium bicarbonate, applied in the field of medicine, can solve the problems of inconvenient experimental operation, troublesome raw material synthesis, high production cost, and achieve the effects of convenient raw material source, low price and simplified operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
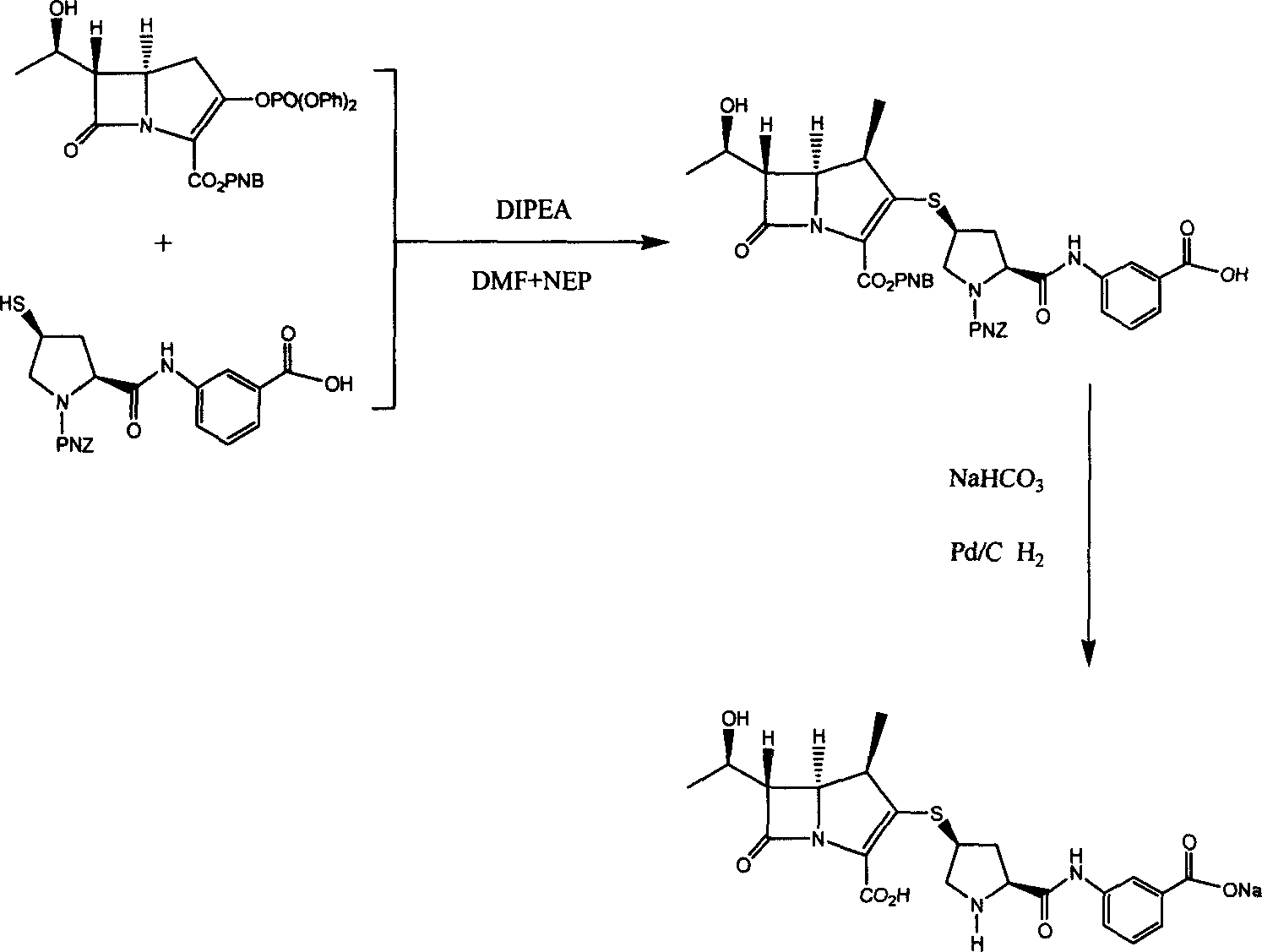
[0027] [4R, 5S, 6S]-3-[[(3S, 5S)-5-[[(3-carboxyphenyl)amino]carbonyl]-3-pyrrolidinyl]thio]-6-[(1R) Preparation of -1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monosodium salt
[0028] [4R, 5S, 6S, 8R)-3-[(diphenylphosphonyloxy)oxy]-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2 0.0 Hept-2-ene-2-carboxylic acid (4-nitrophenyl) methyl ester (2, 0.595g, 1mmol) was dissolved in NMP+DMF (10mL, v / v=3:1), stirred in Add (2S, 4R)-1-(4-nitrobenzyloxycarbonyl)-2-(3-allyloxycarbonylphenylcarbamoyl)pyrrolidin-4-ylthiol (0.445g, 1equiv .) NMP+DMF (10mL, v / v=3:1) solution, transferred to -40°C cold bath, quickly added DIPEA (0.38mL, 2.2equiv.) after 10min, stirred vigorously, and reacted in 4h Finish.
[0029] Add 20 mL of deionized water treated with ultrasound and nitrogen bubbling to the hydrogenation bottle, add anhydrous sodium bicarbonate (84 mg, 1 equiv.), 10% palladium / carbon (0.29 g, 0.2 equiv.) in a nitrogen atmosphere at 0 ° C The above ...
Embodiment 2
[0035] [4R, 5S, 6S]-3-[[(3S, 5S)-5-[[(3-carboxyphenyl)amino]carbonyl]-3-pyrrolidinyl]thio]-6-[(1R) Preparation of -1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monosodium salt
[0036] [4R, 5S, 6S, 8R)-3-[(diphenylphosphonyloxy)oxy]-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2 0.0 Hept-2-ene-2-carboxylic acid (4-nitrophenyl) methyl ester (2, 0.595g, 1mmol) was dissolved in NMP+DMF (10mL, v / v=3:1), stirred in Add (2S, 4R)-1-(4-nitrobenzyloxycarbonyl)-2-(3-allyloxycarbonylphenylcarbamoyl)pyrrolidin-4-ylthiol (0.445g, 1equiv .) NMP+DMF (10mL, v / v=3:1) solution, transferred to -20°C cold bath, quickly added DIPEA (0.38mL, 2.2equiv.) after 10min, stirred vigorously, and reacted in 4h Finish.
[0037] Add 20 mL of deionized water treated with ultrasound and nitrogen bubbling to the hydrogenation bottle, add anhydrous sodium bicarbonate (84 mg, 1 equiv.), 10% palladium / carbon (0.29 g, 0.2 equiv.) in a nitrogen atmosphere at 0 ° C The above ...
Embodiment 3
[0040] [4R, 5S, 6S, 8R)-3-[(diphenylphosphonyloxy)oxy]-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2 0.0 Hept-2-ene-2-carboxylic acid (4-nitrophenyl) methyl ester (2, 0.595g, 1mmol) was dissolved in NMP+DMF (10mL, v / v=3:1), stirred in Add (2S, 4R)-1-(4-nitrobenzyloxycarbonyl)-2-(3-allyloxycarbonylphenylcarbamoyl)pyrrolidin-4-ylthiol (0.445g, 1equiv .) NMP+DMF (10mL, v / v=3:1) solution, transferred to -60°C cold bath, quickly added DIPEA (0.38mL, 2.2equiv.) after 10min, stirred vigorously, and reacted in 8h Finish.
[0041] Add 20 mL of deionized water treated with ultrasound and nitrogen bubbling into the hydrogenation bottle, add anhydrous sodium bicarbonate (0.25 g, 3 equiv.), 10% palladium / carbon (0.15 g, 0.1 equiv.) The above reaction solution was poured under the atmosphere, and kept at 20atm for 11h.
[0042] The palladium / carbon was filtered off, the filtrate was treated with activated carbon in an ice-water bath and a nitrogen atmosphere, and then extracted twic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com