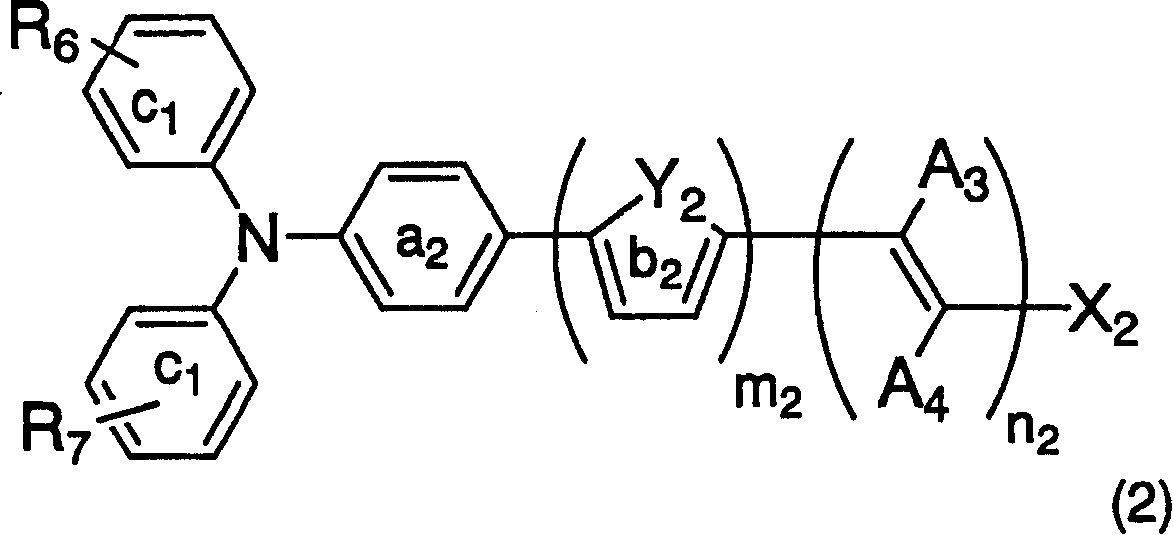
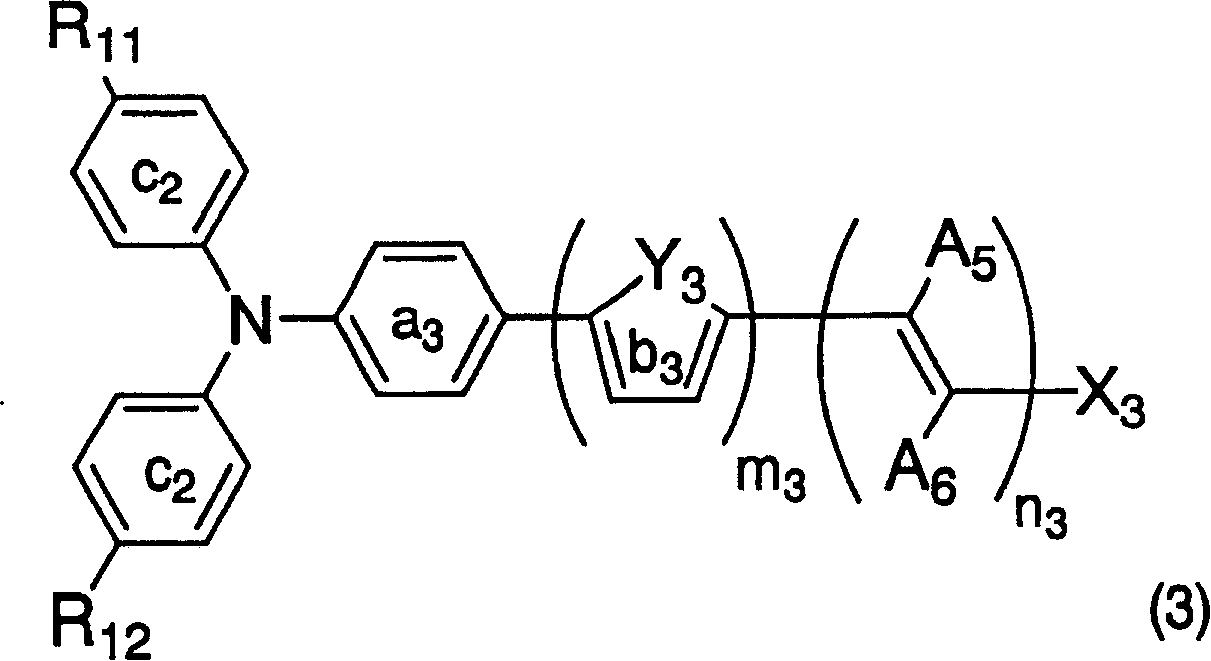
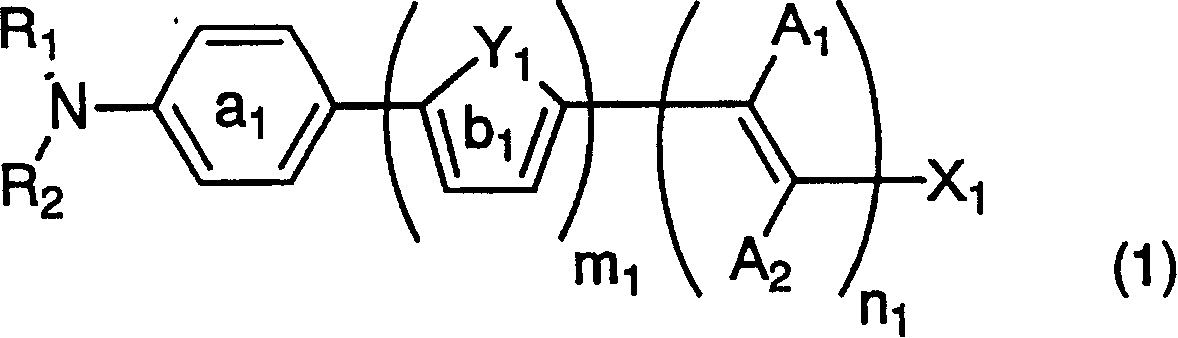Dye-sensitized photoelectric conversion device
A photoelectric conversion and device technology, applied in the fields of photovoltaic power generation, electrical components, semiconductor devices, etc., can solve the problems of lack of practicality, low durability, and high cost of pigments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] 1 part of the following compound (532) and 0.45 parts of methyl cyanoacetate were dissolved in 10 parts of ethanol, and 0.05 parts of anhydrous piperazine was added to the solution. After performing the reflux reaction for 2 hours, the solid obtained by cooling was filtered, washed, and dried. The solid was refluxed in 20 parts ethanol in the presence of 1 part potassium hydroxide for 2 hours. After adding 50 parts of water to the reaction solution, it was neutralized with hydrochloric acid, and the precipitated orange crystals were filtered, washed with water, and recrystallized with ethanol to obtain 0.71 parts of Compound (197) as orange-brown crystals.
[0133] λmax (EtOH: 435nm)
[0134] 1H-NMR (PPM: d6-DMSO): 2.97 (s.CH 3 .6H), 6.77(d.arom.2H), 7.42(d.thio.1H), 7.56(d.arom.2H), 7.66(d.thio.1H), 8.08(s.-CH=.1H)
[0135]
Embodiment 2
[0137] Except that 1 part of compound (532) was replaced with 1.6 parts of the following compound (533), the same treatment as in Example 1 was carried out to obtain 0.98 parts of compound (205) as orange-brown crystals.
[0138] λmax (EtOH: 431)
[0139] 1H-NMR (PPM: d6-DMSO): 6.98 (d.arom.2H), 7.12 (m.arom.6H), 7.37 (m.arom.4H), 7.64 (d.thio.1H), 7.69 (d .arom.2H), 8.00(d.thio.1H), 8.47(s.-CH=.1H)
[0140]
Embodiment 3
[0142]Except that 1 part of compound (532) was replaced with 1.7 parts of the following compound (534), the same treatment as in Example 1 was carried out to obtain 1.23 parts of compound (523) as brown crystals.
[0143] λmax (EtOH: 457nm)
[0144] 1H-NMR (PPM:d6-DMSO): 6.98(d.arom.2H), 7.01-7.20(m.(arom.6H+-CH=.1H)), 7.27-7.44(m.(arom.4H+-CH =.1H)), 7.64(d.thio.1H), 7.68(d.arom.2H), 7.99(d.thio.1H), 8.47(s.-CH=.1H)
[0145]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com