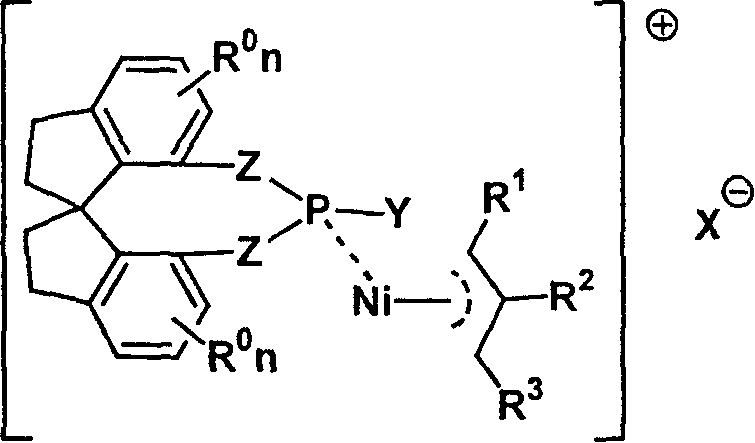
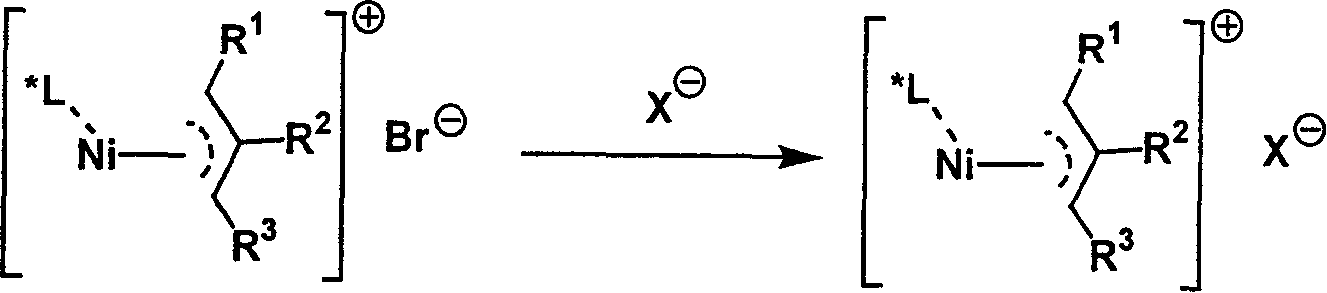
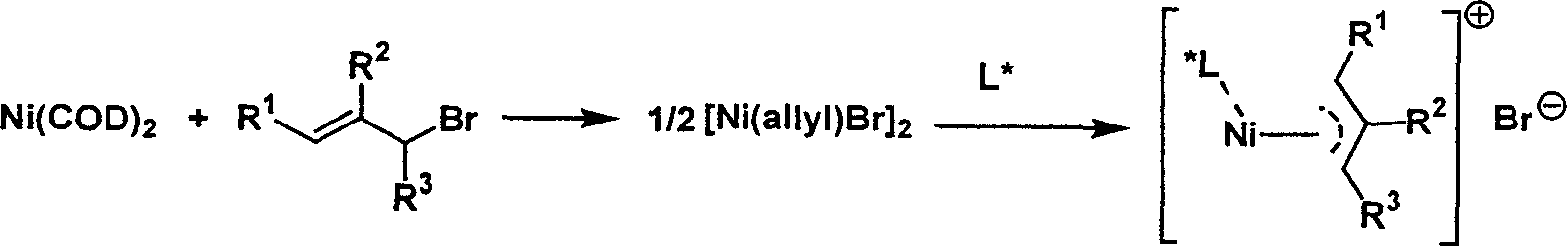Metal complex catalyst of chiral spirocyclo mono-phosphorus (phosphine) ligand and nickel, its prepn. method and application
A metal complex and catalyst technology, which is applied in the field of new nickel metal complex catalysts, can solve the problems of poor reaction results and achieve good chemoselective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: (S, R, R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-N,N-di-2-phenylethylphosphoramidene Preparation of Propyl Nickel Bromide Complex
[0026]
[0027] Under nitrogen atmosphere, the Ni(COD) 2 (137.5mg, 0.5mmol) was added 1mL COD (COD=1,5 cyclooctadiene), stirred for a few minutes, then allyl bromide (63.5mg, 0.525mmol) was added dropwise at 20°C, and stirred for 5~ After 10 minutes, dark blood red allyl nickel bromide dimer was obtained. Add 2 mL of toluene to it for dilution, and then add (S, R, R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-N,N-di-2-phenylethyl A solution of phosphoamidite (252 mg, 0.5 mmol) in 4 mL of toluene was stirred at 20° C. for 2 hours. The resulting orange-red mixture was filtered through celite under nitrogen atmosphere, and the filtrate was vacuum-dried to dry the solvent. The obtained red solid was vacuum-dried for 10 hours to obtain 332 mg of orange-red solid powder with a yield of 97%.
Embodiment 2
[0028] Example 2: (R, R, R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-N,N-di-2-phenylethylphosphoramidene Preparation of Propyl Nickel Bromide Complex
[0029]
[0030] Under nitrogen atmosphere, the Ni(COD) 2 (137.5mg, 0.5mmol) was added 1mL COD (COD=1,5 cyclooctadiene), stirred for a few minutes, then allyl bromide (63.5mg, 0.525mmol) was added dropwise at 20°C, and stirred for 5~ After 10 minutes, dark blood red allyl nickel bromide dimer was obtained. Add 2 mL of toluene to it for dilution, then add (R, R, R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-N,N-di-2-phenylethyl A solution of phosphoamidite (252 mg, 0.5 mmol) in 4 mL of toluene was stirred at 20° C. for 2 hours. The resulting orange-red mixture was filtered through celite under nitrogen atmosphere, and the filtrate was vacuum-dried to dry the solvent. The obtained red solid was vacuum-dried for 10 hours to obtain 335 mg of orange-red solid powder with a yield of 98%.
Embodiment 3
[0031] Example 3: Complexation of (R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-N,N-dimethylphosphoramidite allyl nickel bromide preparation
[0032]
[0033] Under nitrogen atmosphere, the Ni(COD) 2 (137.5mg, 0.5mmol) was added 1mL COD (COD=1,5 cyclooctadiene), stirred for a few minutes, then allyl bromide (63.5mg, 0.525mmol) was added dropwise at 20°C, and stirred for 5~ After 10 minutes, dark blood red allyl nickel bromide dimer was obtained. Add 2 mL of toluene to it for dilution, and then add (R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-N,N-dimethylphosphoramidite (163 mg, 0.5 mmol) in 4 mL of toluene, and continued to stir at 20°C for 2 hours. The resulting orange-red mixture was filtered through celite under nitrogen atmosphere, and the filtrate was vacuum-dried to dry the solvent. The obtained red solid was vacuum-dried for 10 hours to obtain 247 mg of yellow-green solid powder with a yield of 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com