Singlet oxygen europium coordination compound fluorescent probe and application thereof
A technology of europium complexes and fluorescent probes, which is applied in the field of new 1O2 europium complex fluorescent probes, can solve the problems of poor water solubility and unfavorable measurement of probes, and achieve high sensitivity, good selectivity, and high stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
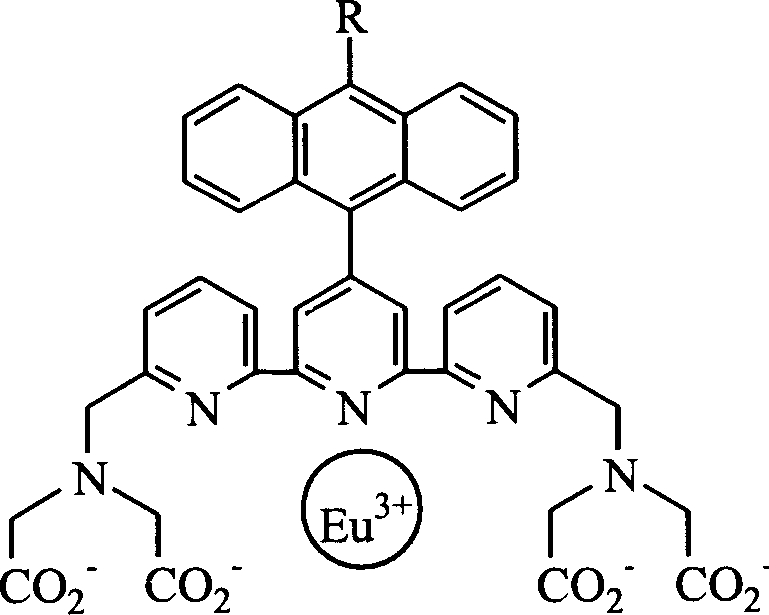
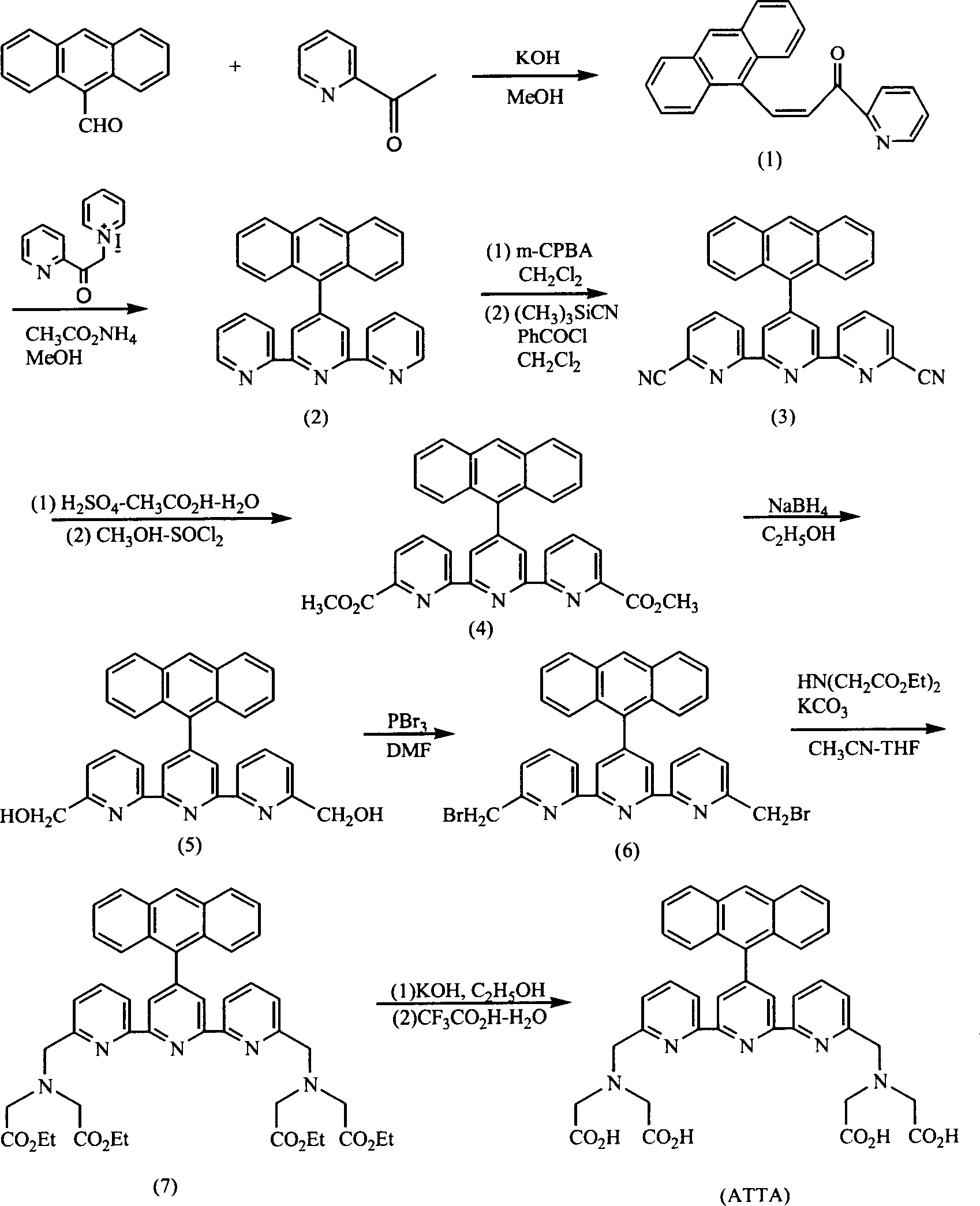
[0026] Example 1. Synthesis of ligand [4'-(9-anthracenyl)-2,2':6'2"-biteropyridine-6,6"-dimethylamine]tetraacetic acid (ATTA for short).
[0027] Synthetic route such as figure 2 As shown, the substrate operation process is as follows.
[0028] (1) Synthesis of (E)-3-(9-anthracenyl)-1-(2'-pyridyl)-2-propenone (compound 1)
[0029] 10.31 grams of 9-anthral (50mmol) and 2.81 grams of KOH (50mmol) were mixed and dissolved in a mixed solution composed of 200ml of methanol and 40ml of water; after stirring for 30 minutes, 6.06 grams of 2-acetylpyridine (50mmol) was slowly added dropwise to The reaction system was stirred at room temperature for 24 hours; the precipitate was collected by filtration, and the crude product was recrystallized from ethanol; 12.77 g of the target compound was obtained, with a yield of 82.6%. 1 H NMR (CDCl 3 ) measurement results: δ=8.48-8.54 (m, 5H); 7.92 (m, 1H); 8.03 (d, J=8.4Hz, 2H); 8.27-8.31 (m, 2H); 8.37 (d, J=8.0 Hz, 2H); 8.48 (s, 1H); 8.70 (d...
Embodiment 2
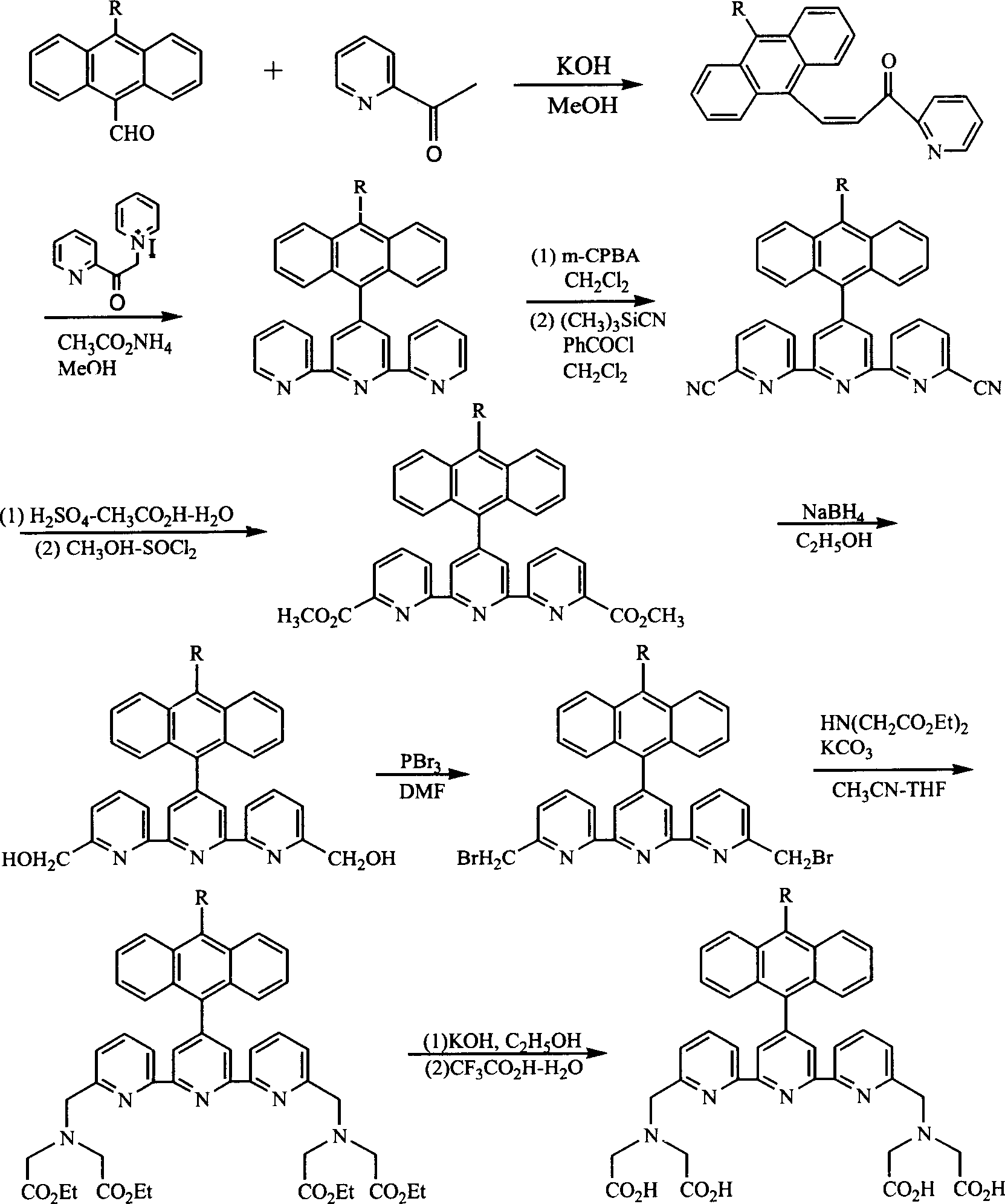
[0045] Example 2. 10-methyl-9-anthracenyl and 10-phenyl-9-anthracenyl substituted [2,2':6',2"-biterpyridine-6,6"-dimethylamine] tetra Synthesis of acetic acid ligand
[0046] Synthetic route such as image 3 Shown, synthetic operation method is identical with embodiment 1.
Embodiment 3
[0047] Example 3. Ligands ATTA and Eu 3+ complexes (abbreviated as ATTA-Eu 3+ )and 1 o 2 Reaction
[0048] 36mg ATTA (0.05mmol) and 18mg EuCl 3 ·6H 2 O (0.05mmol) dissolved in NaHCO at pH 10.5 3 -NaOH buffer solution, after stirring at room temperature for 2 hours, add 1.2g Na 2 MoO 4 2H 2 O (5 mmol) and 500 μl 30% H 2 o 2 , and stirred for another 30 minutes; observe the change in the fluorescence intensity of the reaction solution, and continue to add H 2 o 2 , until the fluorescence intensity of the reaction solution no longer changes; adjust the pH value of the reaction solution to about 3 with HCl, collect the precipitate by filtration, wash with water and dry in vacuum; ATTA-Eu 3+ and 1 o 2 The internal oxide generated by the reaction (referred to as EP-ATTA-Eu 3+ ) determined by ESI-MS: ESI-MS: m / z (%): 882 (10) [M - -H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com