Processes for making thiazolidinedione derivatives and compounds thereof
A compound and alkyl technology, applied in the field of producing thiazolidinedione derivatives, can solve the problems of troublesome purification, low chemical yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
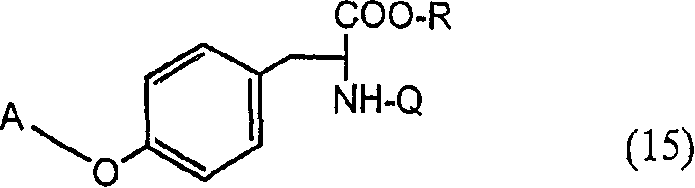
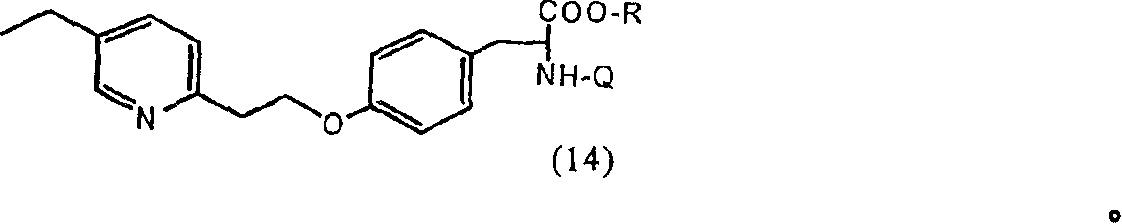
[0129] Preparation of 2-amino-3-{4-[2-(5-ethyl-pyridin-2-yl)-ethoxy]-phenyl}- by alkylation of L-tyrosine sodium salt in DMSO Propionic acid (compound (11), R=H)
[0130] 10 g of L-tyrosine was dissolved in 43 mL of 1M NaOH, 245 mL of dimethylsulfoxide was added, followed by 14 g of 2-(5-ethyl-pyridin-2-yl)-ethyl methanesulfonate (Preparation 3). The reaction mixture was stirred for more than 72 hours, the solvent was removed under vacuum and the residue was dissolved in 100 mL of water. The aqueous solution was neutralized with 6N hydrochloric acid. The precipitate was filtered off and washed with water. Recrystallization from hot methanol-water solution gave 3.1 g of 2-amino-3-{4-[2-(5-ethyl-pyridin-2-yl)-ethoxy]-phenyl}-propionic acid (R =H).
Embodiment 2
[0132] Preparation of 2-amino-3-{4-[2-(5-ethyl-pyridin-2-yl)-ethoxy]-phenyl}- by alkylation of L-tyrosine lithium salt in DMSO Propionic acid (compound (11), R=H)
[0133] 14 g of L-tyrosine were dissolved in a mixture of 12 g of lithium hydroxide and 120 mL of water. Then, 400 mL of dimethylsulfoxide was added, followed by a solution of 23.5 g of 2-(5-ethyl-pyridin-2-yl)-ethyl methanesulfonate (Preparation 3) in 100 mL of toluene. The reaction mixture was stirred at ambient temperature for 48 hours, after which time it was extracted 5 times with 20 mL of toluene. The pH of the dimethylsulfoxide layer was adjusted to 8 with 6N aqueous hydrochloric acid (1:1). The mixture was stirred, the precipitate (8.9 g lithium salt of L-tyrosine) was filtered off, and the filter cake was washed with hot ethanol. Ethanol was evaporated and the residue was added to the filtrate. The filtrate was acidified to pH 2 with hydrochloric acid and the solvent was removed under vacuum at 50°C. T...
Embodiment 3
[0135] Preparation of 2-amino-3-{4-[2-(5-ethyl-pyridin-2-yl)-ethoxy]-benzene by alkylation of L-tyrosine benzyltrimethylammonium salt in DMSO Base}-propionic acid (compound (11), R=H)
[0136] Mix 10 g of L-tyrosine with 25 mL of 40% benzyltrimethylammonium hydroxide in methanol. The mixture was heated and methanol was removed under vacuum. Then, 50 mL of dimethyl sulfoxide was added and the mixture was heated until all solids were dissolved (90°C). The solution was allowed to cool and an additional 3.0 g of sodium hydride was added. Then, a solution of 2.3 g of 2-(5-ethyl-pyridin-2-yl)-ethyl methanesulfonate (Preparation 3) in dimethylsulfoxide and 2.5 g of solid sodium hydride were added portionwise over 6 hours. The reaction mixture was stirred overnight at ambient temperature. Then, 150 mL of acetone was added, and the precipitate was filtered off, dissolved in 50 mL of water and acidified with hydrochloric acid. The solution was extracted with ethyl acetate, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com