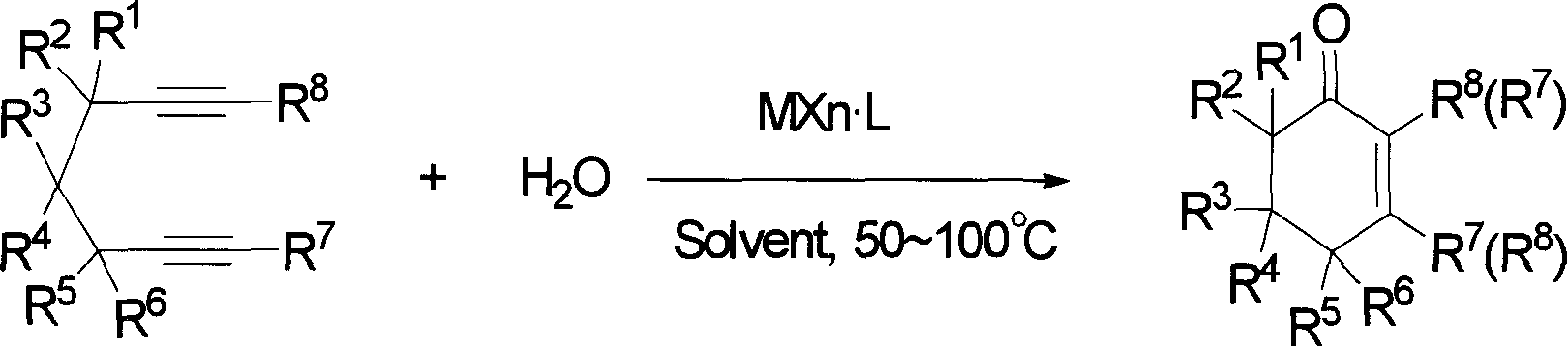2-cyclohexenones and process for preparing same
A technology for cyclohexenones and compounds, which is applied in the field of 2-cyclohexenones and their preparation, can solve problems such as synthesis limitations and restrictions, and achieve good yields, easy operation, and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
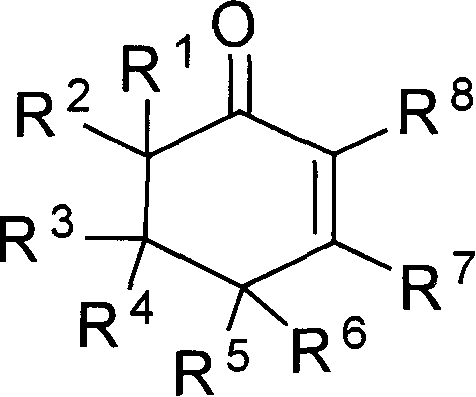
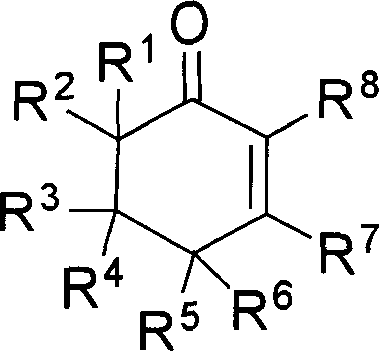
[0019] Embodiment 1: the preparation of 3-methyl-5,5-dimethyl carboxylate-2-cyclohexenone
[0020] Dimethyl 2,2-dipropargyl malonate (104.1 mg, 0.5 mmol), methyl gold complex (MeAuPPh 3 , 5mg, 0.01mmol), trifluoromethanesulfonic acid (100μL, 0.5mmol), water (100μL, 5.0mmol) and n-octane (50μL, internal standard substance for GC analysis) were mixed in methanol (2.0mL), and The mixed solution was heated at below 50° C. for 1 hour to react. After the reaction, according to gas chromatography analysis, the target compound was confirmed with a yield of 94%. Then, the reaction mixture was subjected to column chromatography (silica gel column; developing solvent: petroleum ether: ethyl acetate=5:1; R f =0.3) Separation and purification to obtain the target compound 95mg (yield 84%), and carried out boiling point, NMR, IR, mass analysis and elemental analysis, the results are as follows: Bp: 120 ° C (5mm Hg); 1 H NMR (499.10MHz, CDCl 3 ): δ2.01(s, 3H), 2.87(s, 2H), 2.90(s, 2H), 3....
Embodiment 2
[0021] Example 2: Preparation of 3-methyl-5,5-carbomethoxy-2-cyclohexenone
[0022] The operation was referred to Example 1, except that heterophosphotungstic acid was used instead of trifluoromethanesulfonic acid to obtain 55 mg of the target compound (yield 48%).
Embodiment 3
[0023] Example 3: Preparation of 3-methyl-5,5-carbomethoxy-2-cyclohexenone
[0024] Refer to Example 1 for the operation, except that heterophosphomolybdic acid was used instead of trifluoromethanesulfonic acid to obtain 42 mg of the target compound (yield 37%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com