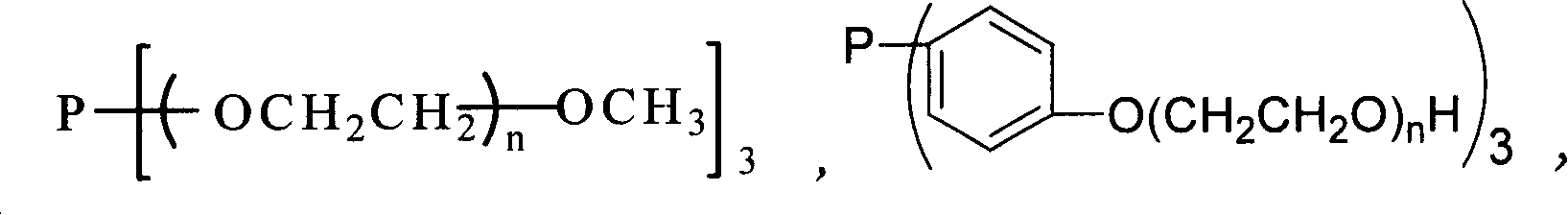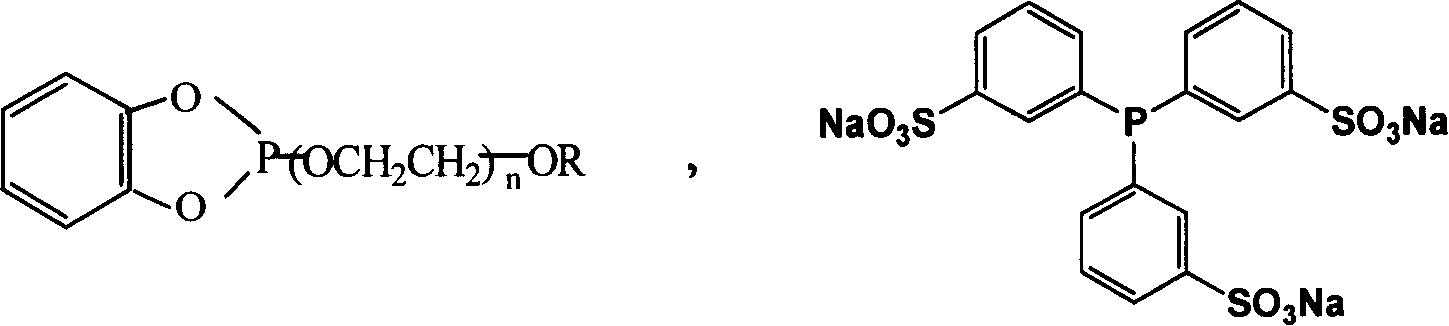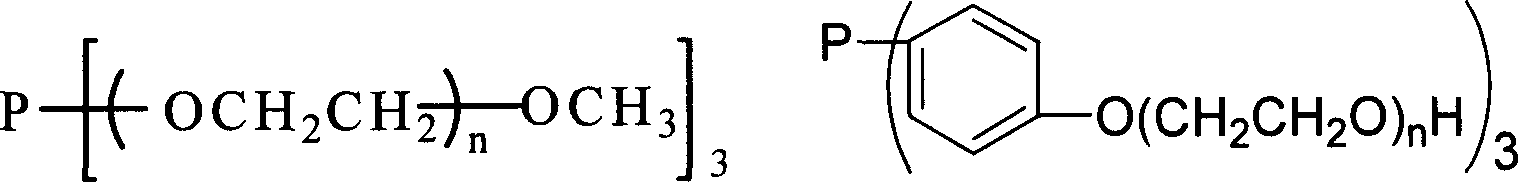Prepn. of high carbon aldehyde by formylating high carbon olefinic hydrogen in temp ion liquid two-phase system
An ionic liquid and system technology, applied in the preparation of carbon monoxide reaction, organic compound/hydride/coordination complex catalyst, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] TPPTS / Rh Catalyzed Temperature-Controlled Ionic Liquid / Organic Biphasic Hydroformylation of Cyclohexene
[0023] The following molar ratios of ionic liquid, Rh(CO) 2 Add acac, TPPTS, organic solvent toluene, n-heptane, cyclohexene, and internal standard decane into a 75mL stainless steel autoclave. Tighten the kettle and check for leaks, replace it with 2.0MPa nitrogen four times, and then charge the synthesis gas at the pressure required for the reaction (CO / H 2 = 1:1). At a temperature of 120°C, a pressure of 5.0 MPa, [C=C] / Rh=1500 (molar ratio), TPPTS / Rh=13 (molar ratio), toluene: n-heptane: the molar ratio of ionic liquid is 18.85:8.89 : 1.05, after 4-10 hours of reaction, take out the reactor and cool to room temperature, under the protection of nitrogen, separate the upper organic phase, and analyze the reaction by GC, which can obtain 93% conversion rate and 95% aldehyde selectivity.
Embodiment 2
[0025] TPPTS / Rh Catalyzed Temperature-Controlled Ionic Liquid / Organic Biphasic 1-Dodecene Hydroformylation
[0026] The following molar ratios of ionic liquid, RhCl 3 ·3H 2 Add O, TPPTS, toluene, n-heptane, decane (internal standard), and 1-dodecene into a 75mL stainless steel autoclave. Tighten the kettle and check for leaks, replace it with 2.0MPa nitrogen four times, and then charge the synthesis gas at the pressure required for the reaction (CO / H 2= 1:1). At a temperature of 110°C, a pressure of 5.0MPa, [C=C] / Rh=1500 (molar ratio), TPPTS / Rh=16 (molar ratio), toluene: n-heptane: the molar ratio of ionic liquid is 18.85:8.89 : 1.05, after 4-10 hours of reaction, take out the reactor and cool to room temperature, under the protection of nitrogen, separate the upper organic phase, GC analysis reaction, can obtain 99% conversion rate and 98% aldehyde selectivity.
Embodiment 3
[0028] Hydroformylation of Cyclohexene Catalyzed by TMPGP / Rh Complex
[0029] The following molar ratios of ionic liquid, Rh(CO) 2 Add acac, TMPGP, organic solvent toluene, n-heptane, cyclohexene, and internal standard decane into a 75mL stainless steel autoclave. Tighten the kettle and check for leaks, replace it with 2.0MPa nitrogen four times, and then charge the synthesis gas at the pressure required for the reaction (CO / H 2 = 1:1). At a temperature of 120°C, a pressure of 5.0 MPa, [C=C] / Rh=1500 (molar ratio), TPPTS / Rh=13 (molar ratio), toluene: n-heptane: the molar ratio of ionic liquid is 18.85:8.89 : 1.05, after 4-10 hours of reaction, take out the reactor and cool to room temperature, under the protection of nitrogen, separate the upper organic phase, GC analysis reaction, can obtain 99% conversion rate and 99% aldehyde selectivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com