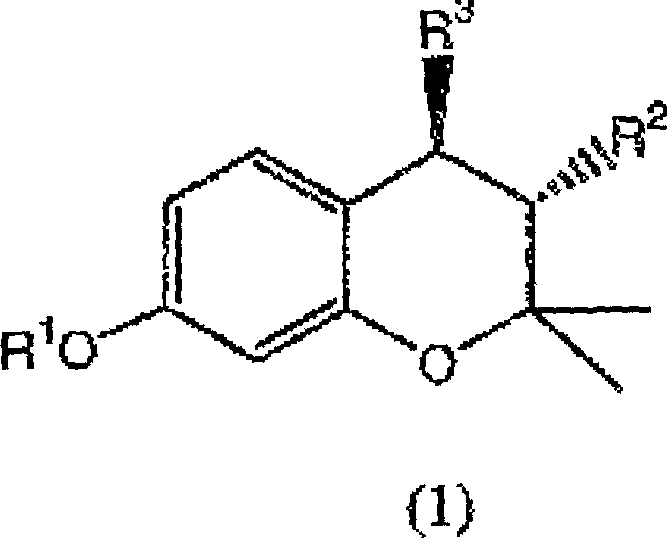
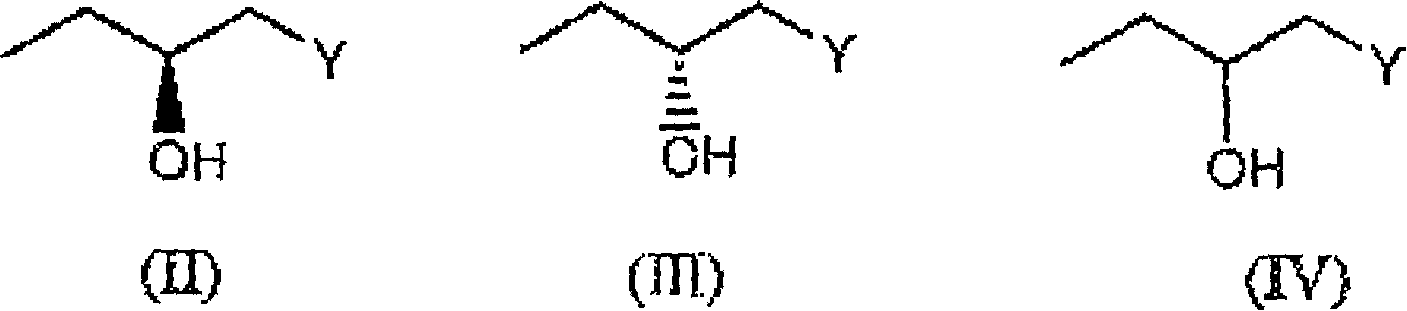
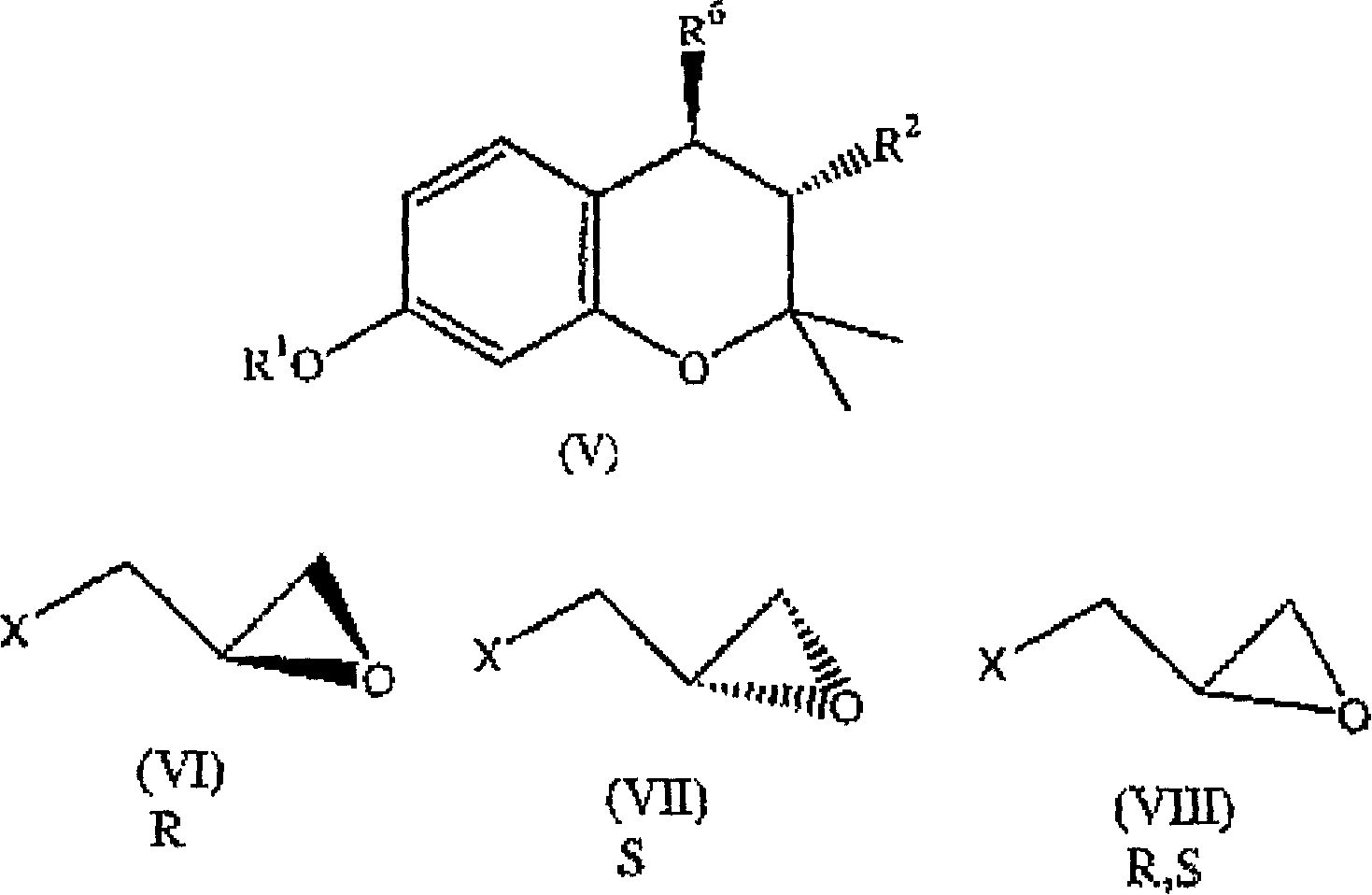
(3R,4R)-trans-3,4-diarylchroman derivatives with estrogenic activity
A chroman and dihydropyran technology, which can be used in sexual diseases, organic chemistry, drug combination, etc., and can solve problems such as deep vein thrombosis and pulmonary embolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] 3R,4R-trans-2,2-dimethyl-3-phenyl-4-(4-[{2R)-(2,3-epoxy-propoxy)-phenyl]-7-methanol Oxychroman (IXa: R 1 = methyl, R 2 = phenyl):
[0268] In anhydrous dimethylsulfoxide (DMSO) (10ml), 3R,4R-trans-2,2-dimethyl-3-phenyl-4-[4-hydroxyphenyl]-7-methoxy A mixture of phenylchroman (0.7g, 1.94mmol), anhydrous potassium carbonate (2.5g, 18mmol) and R(-)epichlorohydrin (0.4ml, 5.11mmol) was stirred at 65°C for 10 hours. The reaction mixture was poured into ice water and extracted with ethyl acetate, washed with water, dried over anhydrous sodium sulfate and concentrated to give an oil, which was crystallized from benzene-hexane to give the target product.
[0269] Yield: 0.6 g (74.18%), (melting point) m.p.: 120°C, [α] D 20 (C=1, methanol): -201.96.
[0270] Infrared spectrum (IR) (KBr, cm -1 ): 1454, 1506, 1585, 1616 (ArH), 1217 (OMe), 2933 (CH), 1382 (gem dimethyl), 758 (C-O).
[0271] 1 H nuclear magnetic resonance (NMR) (δ, CDCl 3 ): 1.2(s, 3H, gem-dimethyl), 1.3(s...
Embodiment 2
[0274] 3R, 4R-trans-2,2-dimethyl-3-phenyl-4-(4-[{2R)-(3-n-butylamino-2-hydroxy}propoxy]-phenyl) -7-Methoxychroman (hydrochloride) (XIa: R 1 = methyl, R 2 = phenyl, Y = butylamino):
[0275] 3R,4R-trans-2,2-dimethyl-3-phenyl-4-(4-[{2R)-(2,3-epoxy-propoxy)-phenyl]-7-methanol A mixture of oxychroman (0.2g, 0.48mmol), n-butylamine (0.5ml, 5.06mmol) and ethanol (15ml) was refluxed for 3 hours. Ethanol was evaporated. The resulting residue was purified by perbasic alumina column using hexane-benzene as eluent. The resulting free base was thus converted to the hydrochloride salt by treatment with ethanol-hydrochloride followed by crystallization from absolute ethanol-ether to give the desired product.
[0276] Yield: 0.160 g, (63.33%) m.p.: 193°C [α] D 20 (C=1, methanol): -160.
[0277] IR (KBr, cm -1 ): 1506, 1585, 1614 (ArH), 1215 (OMe), 2935 (CH), 3417 (OH), 3719 (amine) 1380 (gem-dimethyl).
[0278] 1 HNMR (δ, CDCl 3): 1.2(s, 3H, gem-dimethyl), 1.3(s, 3H, gem-dimethyl...
Embodiment 3
[0282] 3R,4R-trans-2,2-dimethyl-3-phenyl-4-(4-[{2R)-(3-n-butylamino-2-hydroxy}propoxy]-phenyl)- 7-Methoxychroman (citrate) (XIa: R 1 = methyl, R 2 = phenyl, Y = butylamino):
[0283] 3R, 4R-trans-2,2-dimethyl-3-phenyl-4-(4-[{2R)-(2,3-epoxy-propoxy)-phenyl]-7- A mixture of methoxychroman (0.2g, 0.48mmol), n-butylamine (0.5ml, 5.06mmol) and ethanol (15ml) was refluxed for 3 hours. Ethanol was evaporated. The resulting residue was purified by perbasic alumina column using hexane-benzene as eluent. The obtained free base was converted into citrate, and then crystallized from absolute ethanol-ether to obtain the target product.
[0284] Yield: 0.165 g, (63.5%) m.p.: 142 °C [α] D 20 (C=1, methyl): -136.
[0285] IR (KBr, cm -1 ): 1437, 1506, 1589, 1614 (ArH), 1240 (OMe), 2966 (CH), 3429 (OH), 3758 (ammonia) 1380 (gem dimethyl).
[0286] 1 H NMR (δ, CDCl 3 ): 1.2(s, 3H, gem-dimethyl), 1.3(s, 3H, gem-dimethyl), 3.1(d, 1H, monobenzyl H, J=12Hz), 4.2(d, 1H, dibenzyl Base H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com