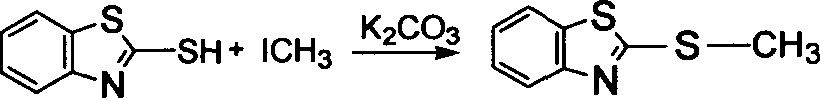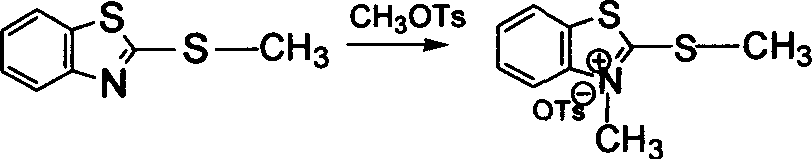Solid phase synthesis process of thiazde orange cyanine dye
A technology for solid-phase synthesis and orange-like cyanine, which is applied in the field of solid-phase synthesis of cyanine dyes, can solve the problems that the solid-phase carrier cannot be cut automatically, and achieve the effects of easy purification, simple separation process, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The solid-phase synthesis of embodiment 1 thiazole orange (TO)
[0030] Step 1: Synthesis of polystyrene-supported 2-benzylbenzothiazole
[0031] Add 2.84g of 2-mercaptobenzothiazole into 30mL of acetone, stir and dissolve, then add 1.38g of potassium carbonate and 1.0g of polystyrene immobilized benzyl chloride, and react at 50°C for 6 hours. After the reaction is complete, unreacted potassium carbonate is removed by filtration, washed with water, dichloromethane and acetone for 3-5 times, and then dried at room temperature to obtain polystyrene-supported 2-benzylbenzothiazole.
[0032] Step 2: Synthesis of polystyrene-supported-3-methyl-2-benzylbenzothiazole p-toluenesulfonate
[0033] The above-mentioned polystyrene-immobilized 2-benzylbenzothiazole and 5.6 g of methyl p-toluenesulfonate were refluxed in toluene at 110 ° C for 72 hours, and then washed with toluene, dichloromethane and acetone for 3 After -5 times, place at room temperature to dry to obtain polysty...
Embodiment 2
[0038] The solid-phase synthesis of embodiment 2 TO-1 (chloro-substituted thiazole orange)
[0039] Step 1: Synthesis of polystyrene-immobilized-5-chloro-2-benzylbenzothiazole
[0040] Add 3.42g of 5-chloro-2-mercaptobenzothiazole into 30mL of acetone, stir to dissolve, add 1.38g of potassium carbonate and 1.0g of polystyrene-supported benzyl chloride, and react at 50°C for 6 hours. After the reaction is complete, unreacted potassium carbonate is removed by filtration, washed with water, dichloromethane and acetone for 3-5 times in sequence, and then dried at room temperature to obtain polystyrene-supported 5-chloro-2-benzylbenzothiazole.
[0041] Step 2: Synthesis of polystyrene-immobilized-5-chloro-3-methyl-2-benzylbenzothiazole p-toluenesulfonate
[0042] The compound obtained in Step 1 and 5.6 g of methyl p-toluenesulfonate were refluxed in toluene at 110°C for 72 hours, then washed with toluene, dichloromethane and acetone for 3-5 times, and then dried at room temperatur...
Embodiment 3
[0047] Example 3 The solid-phase synthesis of TO-2 (nitro-substituted thiazole orange)
[0048] Step 1: Synthesis of polystyrene-supported 5-nitro-2-benzylbenzothiazole
[0049] Add 3.61g of 5-nitro-2-mercaptobenzothiazole into 30mL of acetone, stir to dissolve, add 1.38g of potassium carbonate and 1.0g of polystyrene immobilized benzyl chloride, and reflux at 50°C for 6 hours. After the reaction is complete, unreacted potassium carbonate is removed by filtration, washed with water, dichloromethane and acetone for 3-5 times, and then dried at room temperature to obtain polystyrene-immobilized 5-nitro-2-benzylbenzothiazole .
[0050] Step 2: Synthesis of polystyrene-supported 5-nitro-3-methyl-2-benzylbenzothiazole p-toluenesulfonate
[0051]The above-mentioned polystyrene-immobilized 5-nitro-2-benzylbenzothiazole and 5.6 g of methyl p-toluenesulfonate were refluxed in toluene at 110 ° C for 72 hours, and then successively with toluene, dichloro After washing with methane and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com