Method for preparing oral administered sustained-release peptide micro capsule
A technology of slow-release microcapsules and drugs, which is applied in the fields of pharmaceutical formulations, drug combinations, drug delivery, etc., to achieve good application prospects, mild reaction conditions, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
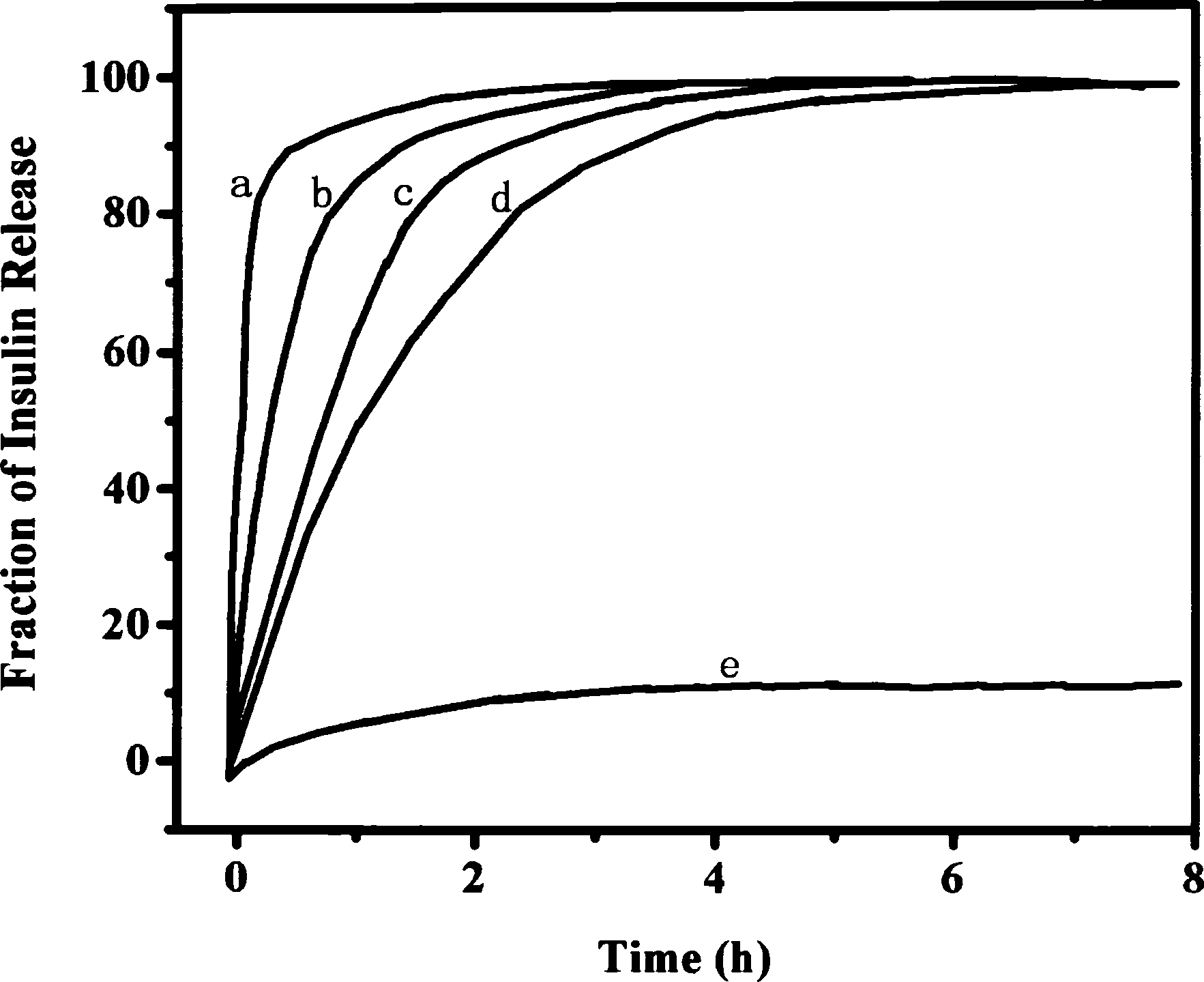
Image
Examples
specific Embodiment approach 1
[0016] Specific implementation mode one: this implementation mode is realized in this way:
[0017] (a) Put the polypeptide drug particles in a 0.01-10M salt solution containing 0.01-100 mg / ml polyanion (pH value is between 1 and 6) for adsorption for 0.1-100 minutes;
[0018] (b) Centrifuge or filter to remove unadsorbed polyanions, and repeat washing several times with 0.01-10M salt solution (pH value between 1 and 6), each washing for 0.1-100 minutes;
[0019] (c) placing the prepared particles in a 0.01-10M salt solution (pH value between 1 and 6) containing 0.01-100 mg / ml polycation for adsorption for 0.1-100 minutes;
[0020] (d) Centrifuge or filter to remove unadsorbed polycations, and repeat washing with 0.01-10M salt solution (pH value between 1 and 6) several times, each time washing for 0.1-100 minutes;
[0021] (e) Steps (a) to (d) are repeated in sequence to obtain polypeptide drug microcapsules assembled with different layers of polymers.
[0022] In this embo...
specific Embodiment approach 2
[0028] Specific implementation mode two: this implementation mode is realized in this way:
[0029] (a) Put the insulin microparticles in a sodium polystyrene sulfonate solution containing 0.01-100 mg / ml (0.01-10 M inorganic salt in a 0.001-100 mM hydrochloric acid solution) for adsorption for 0.1-100 minutes;
[0030] (b) Centrifuge to remove unadsorbed sodium polystyrene sulfonate, repeat washing several times with 0.001-100mM hydrochloric acid solution containing 0.01-10M inorganic salts, and wash for 0.1-100 minutes each time;
[0031] (c) Place the prepared particles in polyallyl ammonium hydrochloride solution containing 0.01-100 mg / ml (0.001-100 mM hydrochloric acid solution containing 0.01-10 M inorganic salt) for adsorption for 0.1-100 minutes;
[0032] (d) Centrifuge to remove unadsorbed polyallyl ammonium hydrochloride, and repeat washing several times with 0.001-100 mM hydrochloric acid solution containing 0.01-10 M inorganic salt, each washing for 0.1-100 minutes;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com