Enhancing transdermal administration of hydrophilic drugs
A drug, hydrophilic technology, used in drug combination, drug delivery, antipyretics, etc., can solve problems such as sensitization, skin irritation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
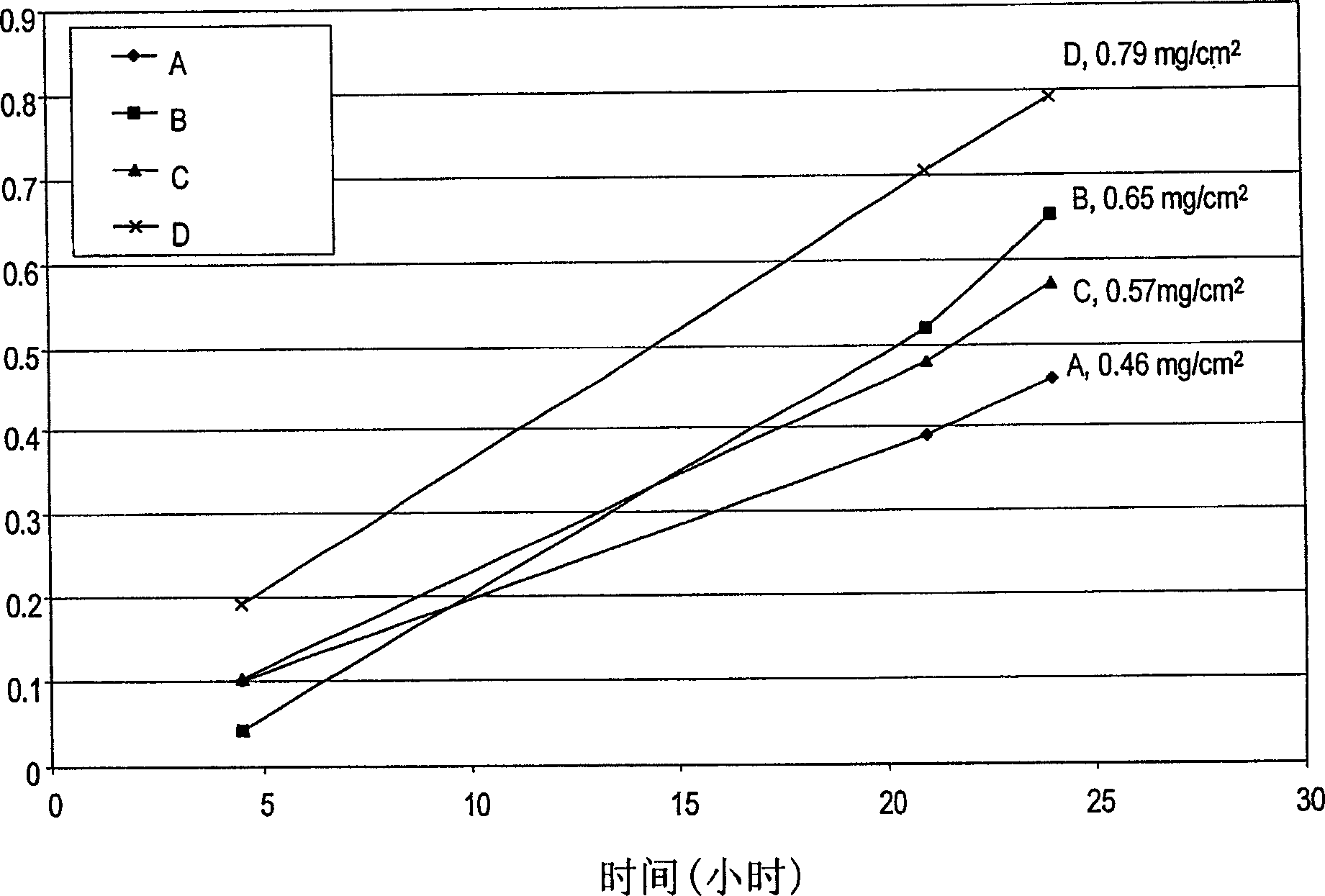
[0087] An in vitro skin permeation study was performed using four solutions of meloxicam, each containing sodium hydroxide and a nitrogenous base. The ingredients used to prepare each formulation are listed in Table 1 along with the actual weight and weight percent of each ingredient in each formulation. Each ingredient was added in the order listed in Table 1. The solution was placed directly on human cadaver skin as described below.
[0088] The in vitro permeability of meloxicam from formulations A, B, C and D was determined by using a diffusion area of 1 cm 2 A Franz-type diffusion cell was evaluated. The volume of the receptor solution was 8 ml. Human cadaver skin was cut to size and placed cuticle-side up on the receptor chamber of the diffusion cell. A donor chamber is placed on top of the stratum corneum, and the pools are clamped together. 50 [mu]l of each meloxicam formulation was added to the stratum corneum sandwiched between the donor and acceptor compartme...
Embodiment 2
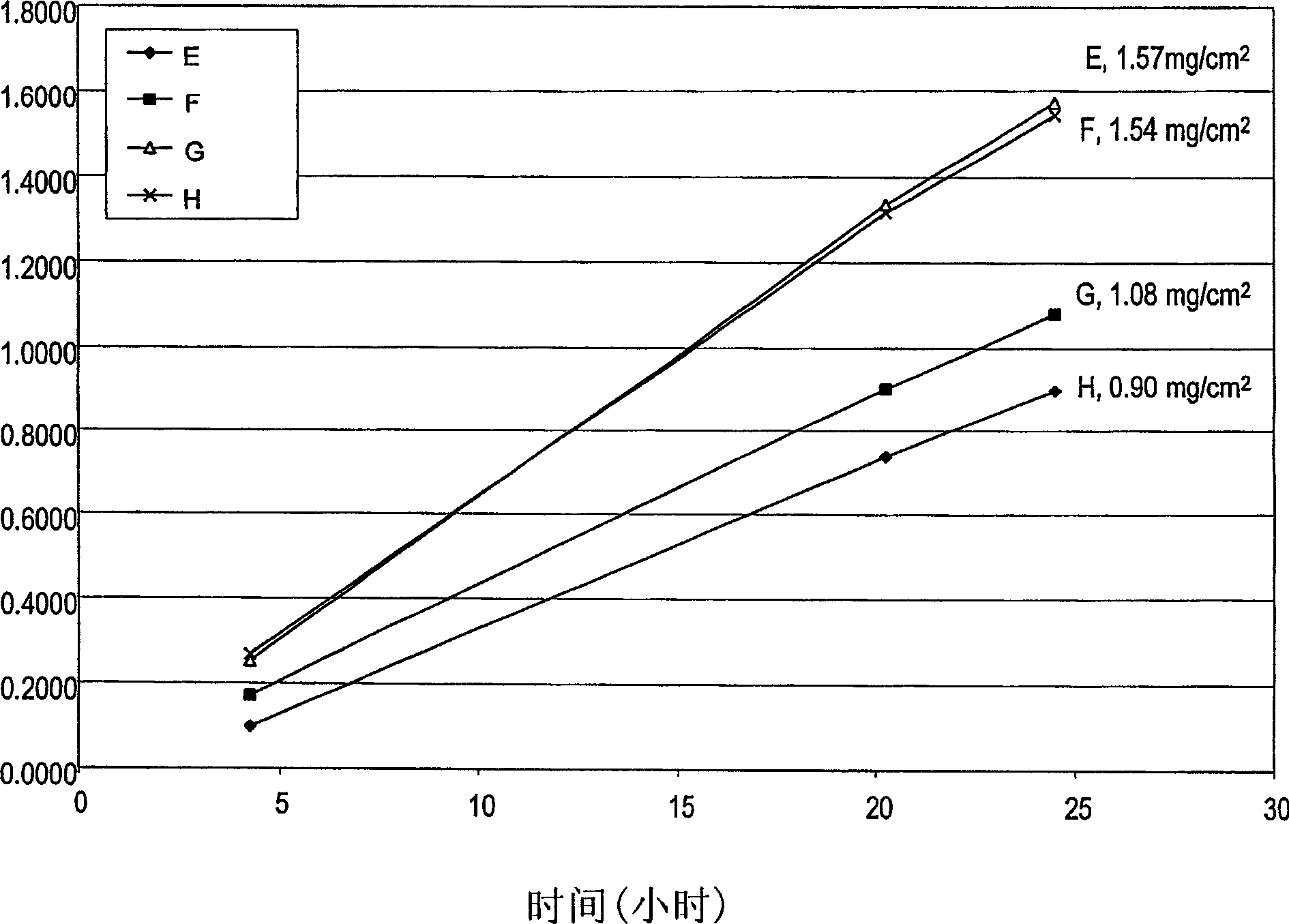
[0093] In vitro skin penetration studies using four diclofenac sodium transdermal drug delivery systems. The ingredients used to prepare each system are listed in Table 2 along with the actual weight and weight percent of each ingredient (based on the total weight of the solution) in each formulation. Each formulation was coated on a release liner and dried in an oven at 65°C for two hours to remove water and other solvents. The dried adhesive coated drug / release liner film was laminated to a liner film, and the liner / adhesive coated drug / release liner sheet was cut into 9 / 16 inch diameter discs. After drying, the theoretical weight percents (calculated assuming that all volatile components were completely removed as precursors during drying) for each component weight of the system are listed in Table 3.
[0094] The in vitro permeability of diclofenac sodium from these discs to human cadaver skin was determined using a diffusion area of 1 cm 2 A Franz-type diffusion cell ...
Embodiment 3
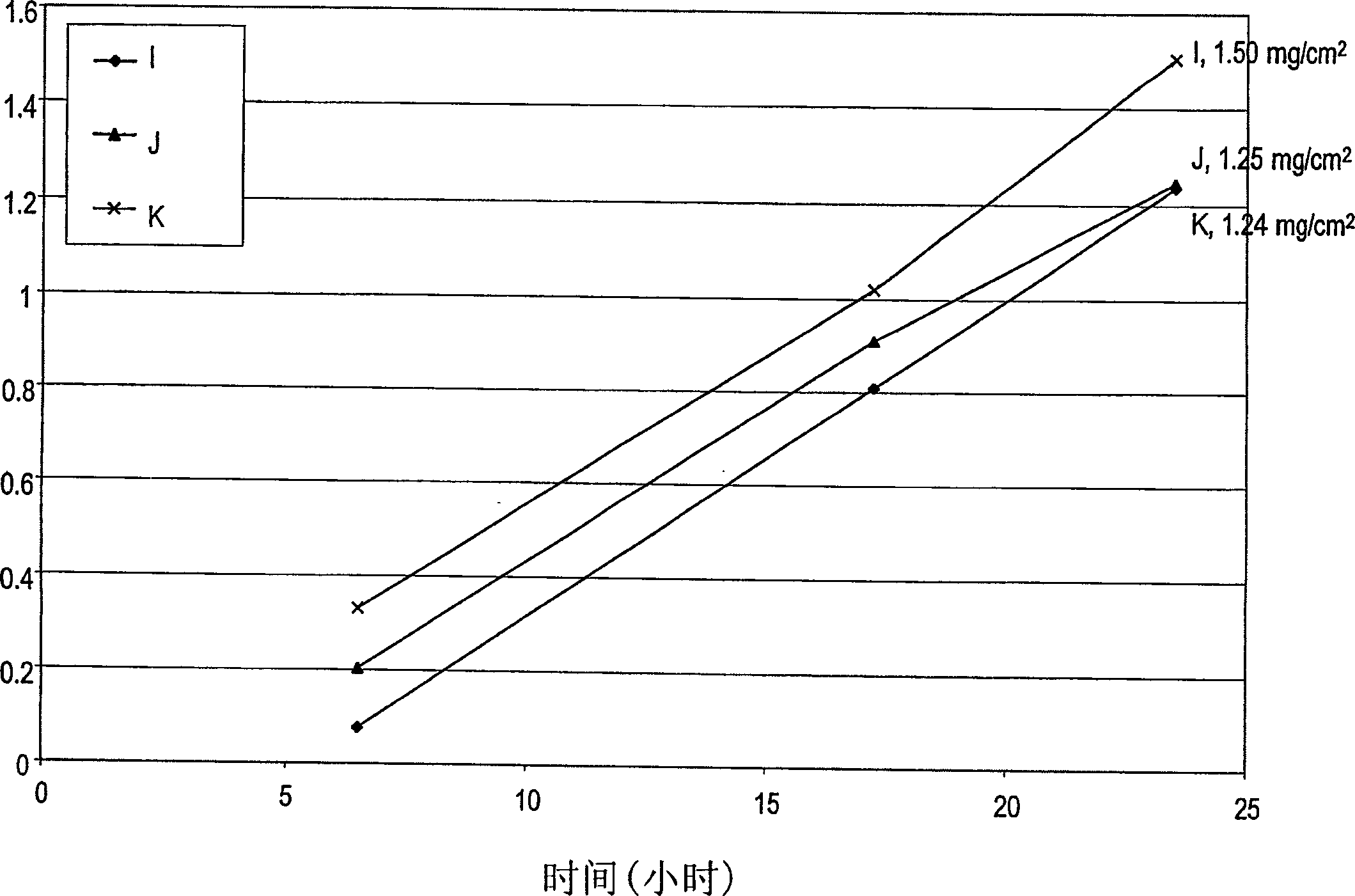
[0101] In vitro skin penetration studies were performed as in Example 1 using three solutions of alendronate sodium, each solution containing sodium hydroxide and a nitrogenous base. The ingredients used to prepare each formulation are listed in Table 4 along with the actual weight and weight percent of each ingredient in each formulation. Each ingredient was added in the order listed in Table 4. The solution was placed directly on human cadaver skin as described below.
[0102] In vitro permeability of alendronic acid from formulations I, J and K using a diffusion area of 1 cm 2 Franz-type diffusion cell evaluation. The volume of the receptor solution was 8 ml. Human cadaver skin was cut to size and placed cuticle-side up on the receptor chamber of the diffusion cell. A donor chamber is placed on top of the stratum corneum, and the pools are clamped together. 50 [mu]l of each meloxicam formulation was added to the stratum corneum sandwiched between the donor and accept...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com