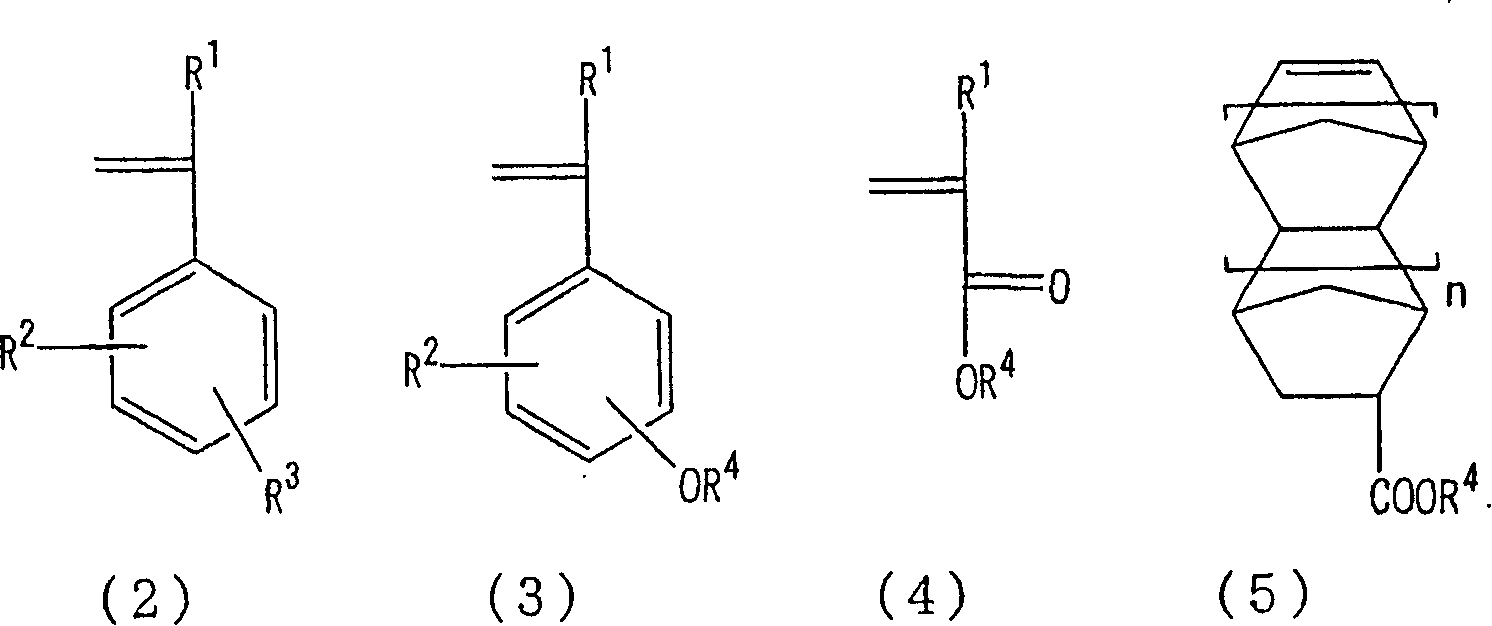
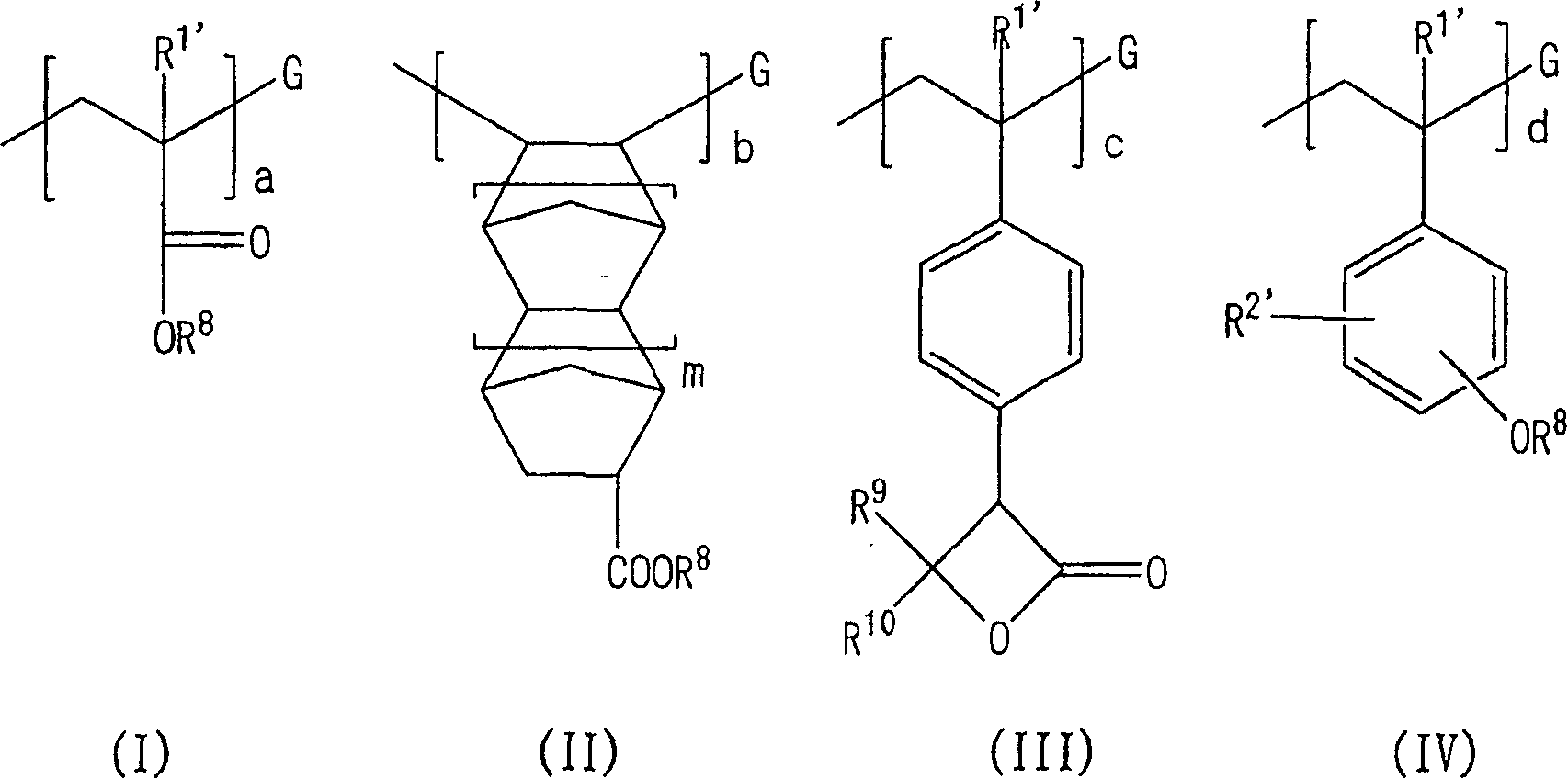
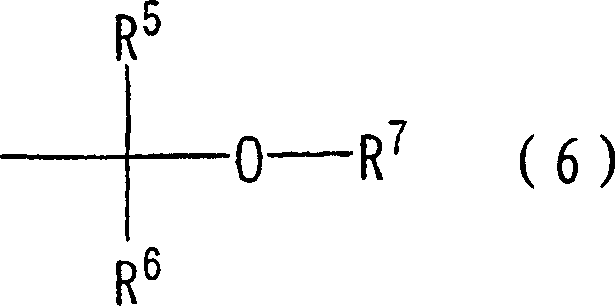Hyperbranched polymer, process for producing the same and resist composition containing the hyperbranched polymer
A hyperbranched polymer and polymer technology, which is applied in the field of resist composition, can solve the problems of molecular design produced by exposure and has not yet been reported, and achieve the effects of excellent film formation, less pollution, and improved alkali solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0204] —Synthesis of hyperbranched polymers—
[0205] In a 50mL reaction vessel, 21mmol of chloromethylstyrene as a reaction monomer, 2.1mmol of 2-bipyridine as a catalyst, 1.1mmol of copper chloride (I) and 8mL of chlorobenzene as a solvent are charged into a 50mL reaction vessel. After replacing the inside of the reaction container, stirring was performed at a temperature of 115° C., and the polymerization reaction was carried out for 1 hour. 50 mL of tetrahydrofuran was added to the reaction liquid to dilute and dissolve the polymer, and the catalyst was removed by filtration through activated alumina. After concentrating the filtrate, 200 mL of methanol was added to precipitate the polymer, and the unreacted monomer and the reaction solvent were removed by removing the supernatant liquid. Then, the operation of dissolving the precipitated polymer in 20 mL of tetrahydrofuran and adding 500 mL of methanol to reprecipitate was repeated twice to synthesize Polymer 1 (yield 75...
Embodiment 2A
[0219] —Synthesis of hyperbranched polymers—
[0220] In embodiment 1, except that the amount of catalyst in the above-mentioned hyperbranched polymer synthesis step is 5 times, the reaction temperature is 125 ℃, and the reaction time is 30 minutes for polymerization, and the same synthetic polymer 2 as in embodiment 1 (received rate of 77%). The weight average molecular weight (Mw) and branching degree (Br) of Polymer 2 were measured in the same manner as in Example 1. The results are shown in Table 1A.
[0221] In addition to using 1 g of polymer 2 as a base polymer in the above-mentioned acid-decomposable group introduction step, using 65 mmol of p-tert-butoxystyrene as a compound containing an acid-decomposable group, and making the reaction time 3 hours , Polymerization and purification with p-tert-butoxystyrene were carried out in the same manner as in Example 1, to synthesize a target acid-decomposable group-introduced hyperbranched polymer.
[0222] The addition amo...
Embodiment 3A
[0224] —Synthesis of hyperbranched polymers—
[0225] In Example 1, except that the amount of catalyst in the above-mentioned hyperbranched polymer synthesis process is 4 times, the reaction temperature is 125 ° C, and the reaction time is 1 hour, the same synthetic polymer 3 as in Example 1 (yield 78 %).
[0226] The weight-average molecular weight (Mw) and branching degree (Br) of Polymer 3 were measured in the same manner as in Example 1. The results are shown in Table 1A.
[0227] In addition to making 1 g of the polymer 3 as the base polymer in the above-mentioned acid-decomposable group introduction step, the p-ethoxyethoxystyrene as the acid-decomposable group-containing compound was 65 mmol, and the reaction time was 3 After 1 hour, polymerization and purification with p-ethoxyethoxystyrene were carried out in the same manner as in Example 1 to synthesize a target acid-decomposable group-introduced hyperbranched polymer.
[0228] The addition amount (introduction am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com