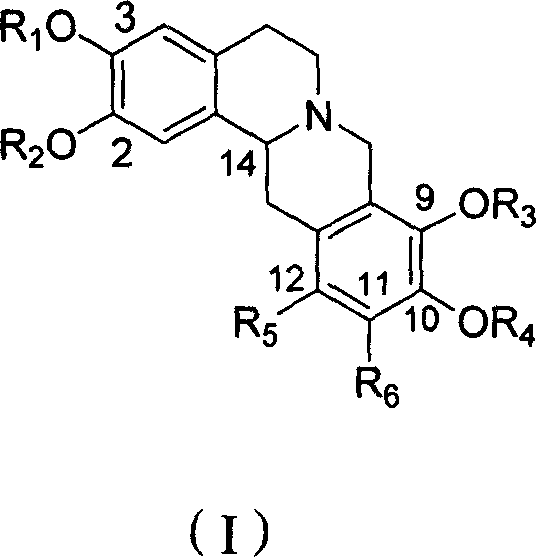
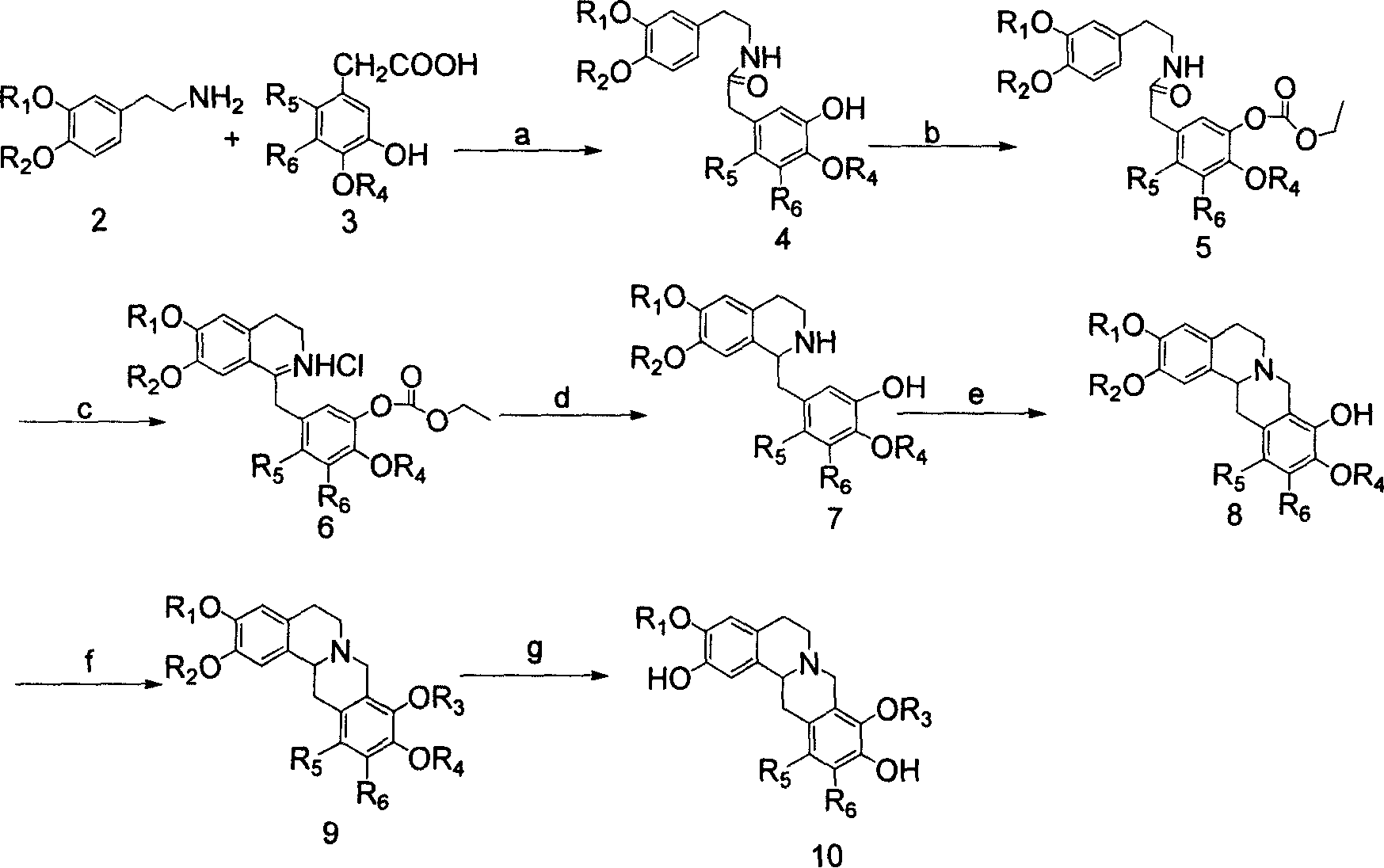
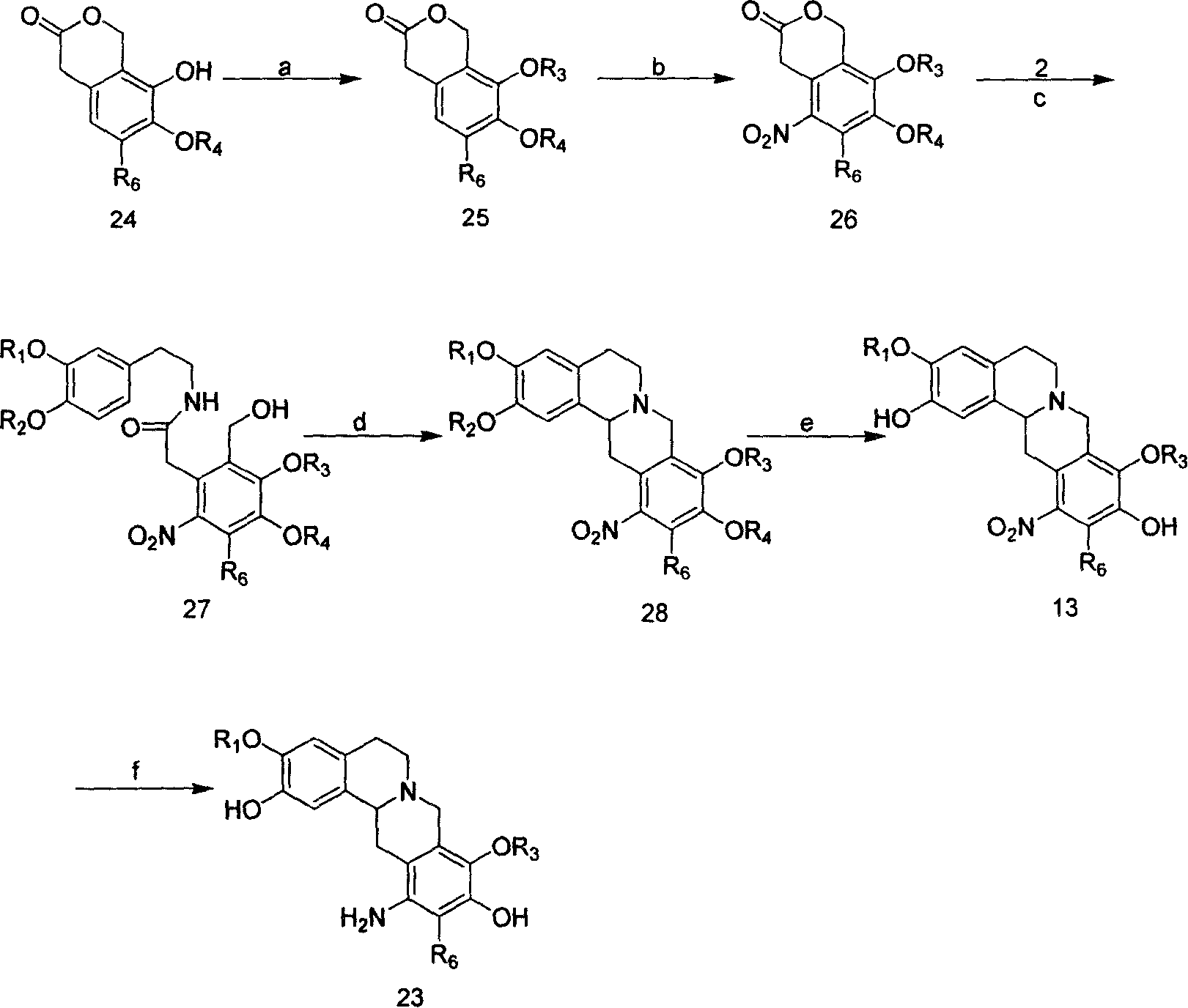
Tetrahydro-proto-berberine compounds, their preparing method, composition and use
A technology of tetrahydroprotoberberine and its compounds, applied in the fields of medicinal chemistry and chemotherapeutics, can solve the problems of uneffective treatment of nervous system diseases and unsatisfactory clinical treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: 3-methoxy-4-benzyloxy-ω-nitrostyrene (compound 1)
[0100] References (a.A.I.Meyeres & Joseph Guiles, Heterocycles, 1989, 28(1), 295-301.b. Paul W.Ford, Mathew R.Narbut, Jack Belli, and Bradley S.Davidson, J.Org.Chem., 1994, 59(20), 5955-5960), starting from vanillin, refluxing with benzyl bromide and potassium carbonate in acetone to obtain a benzyl-protected product, which was refluxed together with nitromethane and ammonium acetate in glacial acetic acid 3-Methoxy-4-benzyloxy-ω-nitrostyrene (1) is produced. M.P.117-119℃. 1 H NMR (CDCl 3 ): δ7.95(d, 1H, J=13.7Hz), 7.52(d, 1H, J=13.5Hz), 7.45-7.31(m, 5H), 7.11-7.02(m, 2H), 6.92(d, 1H, J=8.5Hz), 5.22(s, 2H), 3.93(s, 3H).
Embodiment 2
[0101] Example 2: 3-methoxy-4-benzyloxy-phenethylamine (compound 2)
[0102] Under the protection of argon, lithium aluminum tetrahydride (12.0g, 0.32mol) was suspended in pretreated anhydrous tetrahydrofuran (250ml), and compound 1 (28.5g, 0.10mol) was dissolved in anhydrous tetrahydrofuran (250ml), and stirred , slowly drop the latter into the previous suspension, control the drop rate to prevent the reflux from being too violent, heat and reflux for 2 hours after the dropwise addition, then stir overnight at room temperature, cool with ice water, and slowly drop the mixture under stirring Enter H 2 O (12ml), 15% sodium hydroxide solution (12ml), H 2 O (36ml), the formed turbidity was filtered, the filter cake was repeatedly washed with acetone, the filtrate was concentrated under reduced pressure and dissolved in ether (100-150ml), and the ether solution washed three times with water was dried with potassium carbonate and concentrated to dryness under reduced pressure. , ...
Embodiment 3
[0103] Example 3: 2-Chloro-4-benzyloxy-5-hydroxy-phenylacetic acid (compound 3)
[0104]It is prepared from 3,4-dihydroxybenzaldehyde with reference to literature (B Hegedus, Helv. Chim. Acta., 1963, 46(7), 2604-2612.). mp 100-102°C; 1 H NMR (CDCl 3 ): δ7.42-7.36(m, 5H), 6.90-6.87(m, 2H), 6.74(dd, 1H, J=2.1, 8.1Hz), 5.69(br, 1H), 5.09(s, 2H), 3.56(s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com