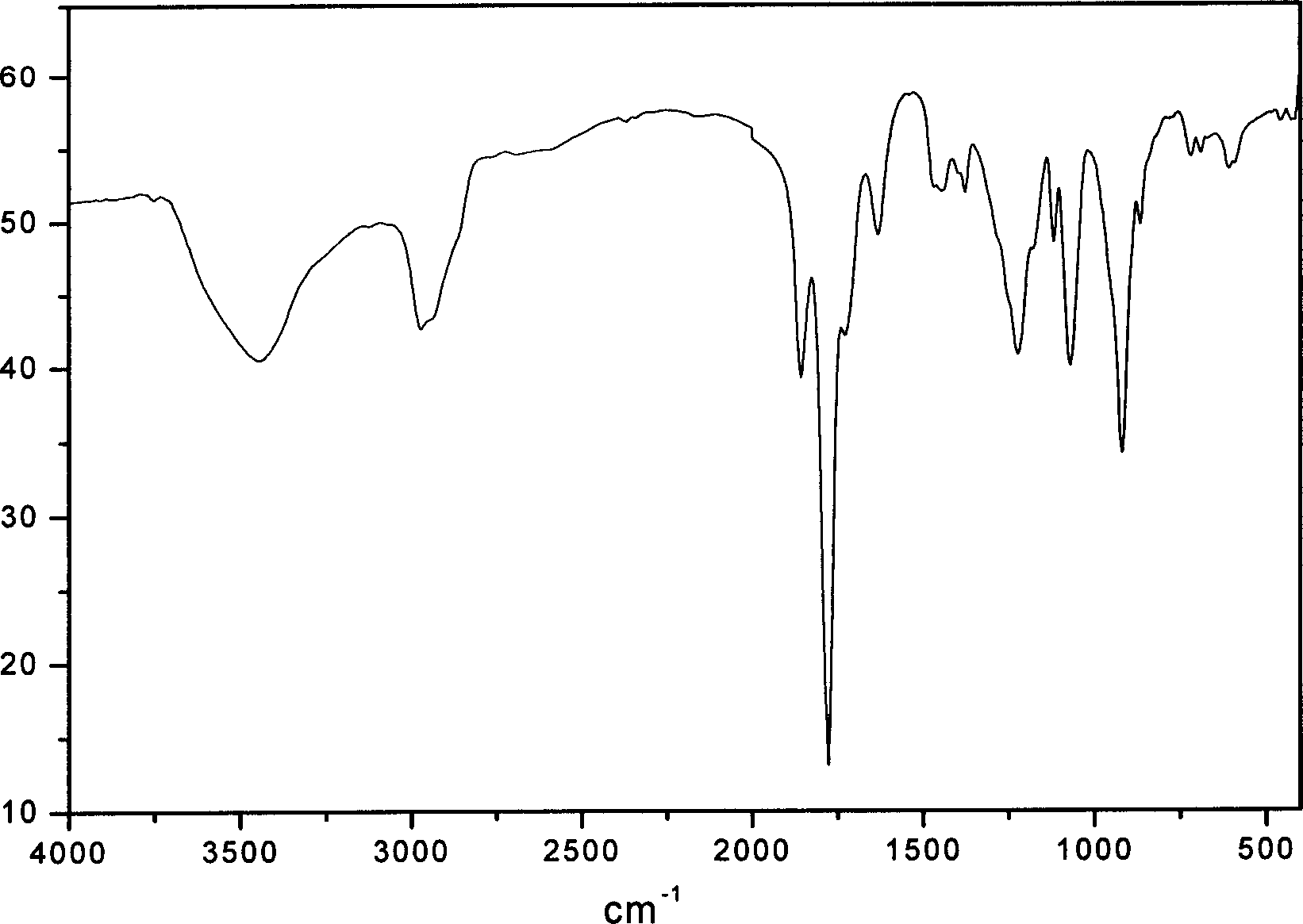
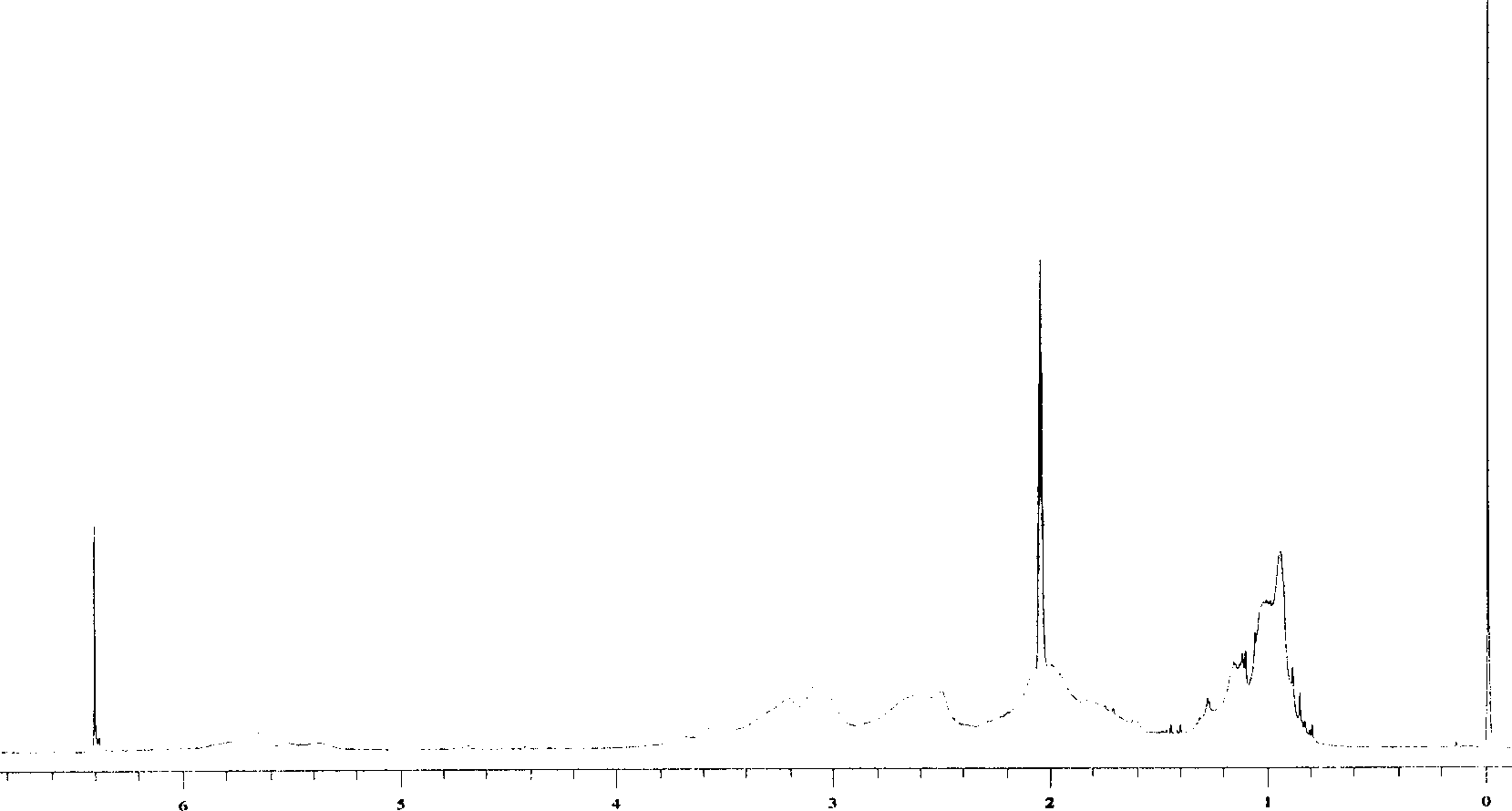
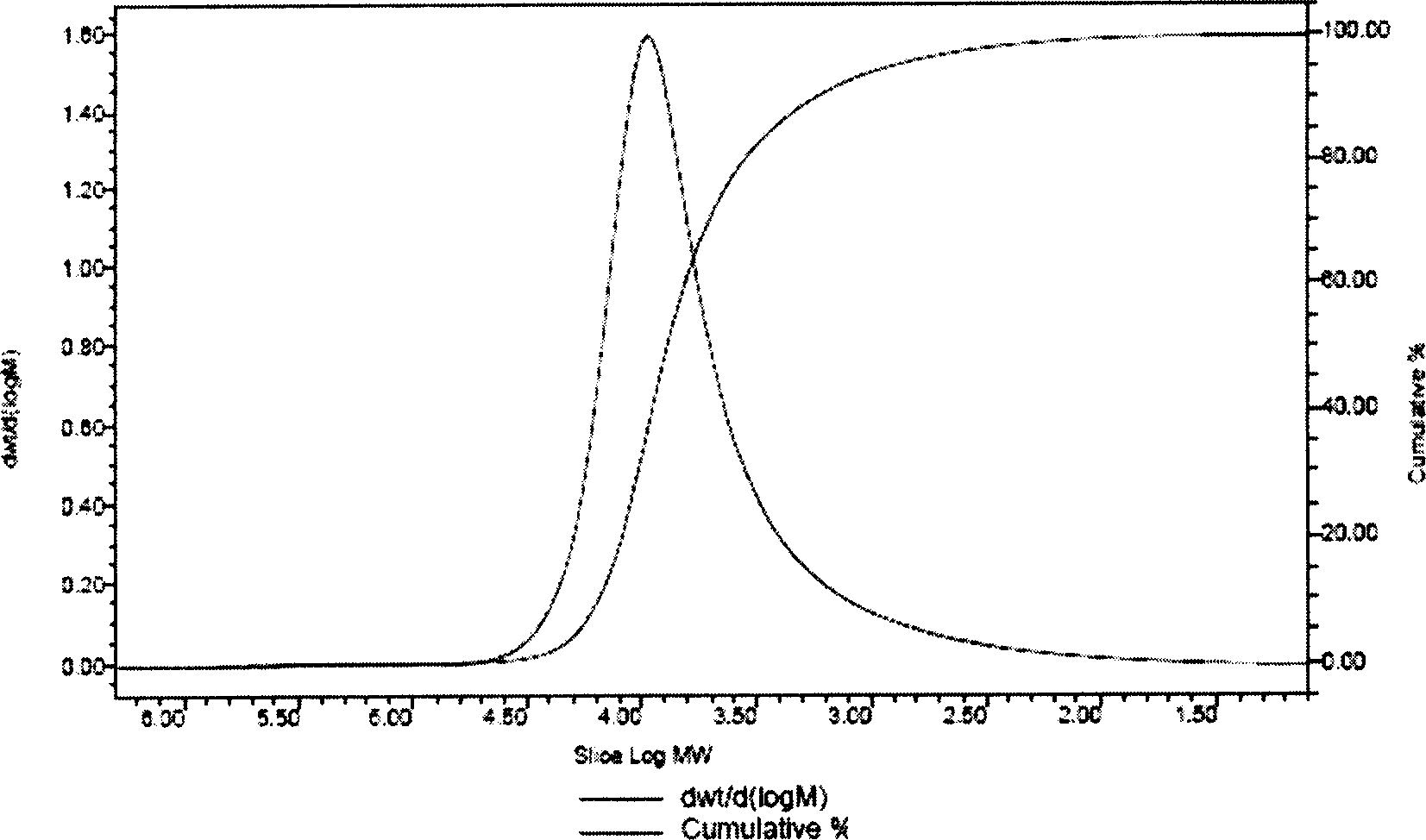
Process for synthesizing beta-pinene/maleic anhydride copolymer
A technology of maleic anhydride and a synthesis method, applied in the field of polymers, can solve the problems of complex post-processing process and high reaction cost, and achieve the effects of convenient post-processing, mild conditions and moderate time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 16.32 grams of β-pinene, 9.80 grams of maleic anhydride (the molar ratio of β-pinene and maleic anhydride is 1.2:1), and 50 ml of dioxygen into a four-necked flask equipped with a thermometer, a condenser, and mechanical stirring. Hexacyclic, add 0.242 grams of benzoyl peroxide when the temperature is raised to 80 ° C, and feed N 2 React under the conditions for 8 hours, then stop the reaction, after cooling to room temperature, pour out the upper layer liquid, add tetrahydrofuran to the lower layer of precipitate, after it is completely dissolved, add ether to re-precipitate the white precipitate, and then suction filter to obtain a white powder The solid was put into a vacuum oven at 50° C. and dried to a constant weight to obtain 10.11 grams of β-pinene / maleic anhydride copolymer.
Embodiment 2
[0023] Adding the mol ratio of β-pinene and maleic anhydride is 1: 1 (13.60 grams of β-pinene, 9.80 grams of maleic anhydride), the consumption of free radical initiator benzoyl peroxide is the amount of the substance of maleic anhydride 3%, other reaction conditions are with embodiment 1, finally obtain the β-pinene / maleic anhydride copolymer of 11.84 grams.
Embodiment 3
[0025] Adding the mol ratio of β-pinene and maleic anhydride is 1: 1.2 (13.6 grams of β-pinene, 11.76 grams of maleic anhydride), the consumption of radical initiator benzoyl peroxide is the amount of the substance of maleic anhydride 5%, other reaction conditions are with embodiment 1, finally obtain the β-pinene / maleic anhydride copolymer of 13.76 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com