Treatment strategies for immune-response disorders by using Fc receptor polymorphisms as diagnostics
An immunotherapeutic, individual technology for use in predictive medicine that addresses issues such as reduced ability to interact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0196] The art provides basic guidance for the preparation and use of polypeptide variants. During the preparation of IL-2 variants, those skilled in the art can easily determine which modification of the nucleotide or amino acid sequence of the native protein will result in the production of IL-2 suitable for use as a therapeutically active component in the pharmaceutical composition of the method of the present invention. Variants.
[0197] IL-2 or variants thereof for use in the methods of the invention may be of any origin, but are preferably produced recombinantly. "Recombinant IL-2" or "recombinant IL-2 variant" refers to a biological activity comparable to that of native sequence IL-2 and described by, for example, Taniguchi et al., (1983), Nature, 302:305-310 and Devos, (1983) , Interleukin-2 or its variants prepared by recombinant DNA technology described in Nucleic Acids Research, 11: 4307-4323; or IL-2 changed by mutation as described in Wang et al., (1984), Scienc...
Embodiment 1
[0228] Example 1: Materials and methods
[0229] A.IL-2
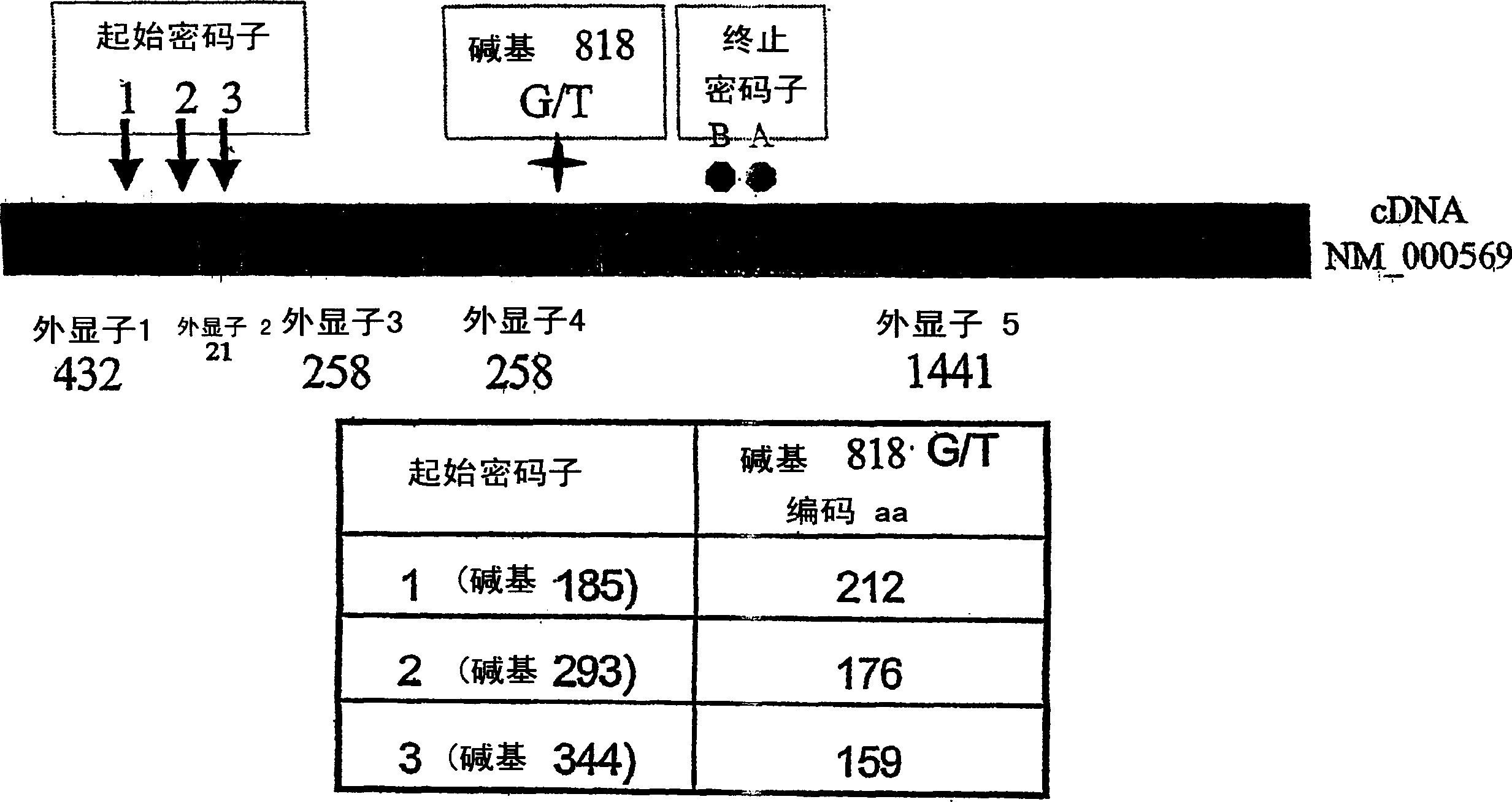
[0230] The IL-2 formulation used was manufactured by Chiron Corporation of Emeryville, California under the trade name Proleukin . IL-2 in this formulation is a recombinantly produced, unglycosylated IL-2 mutein called aldesleukin, which has the initial alanine residue deleted and cysteine residue 125 replaced with a serine residue ( Known as de-alanyl-1, serine-125 human interleukin-2) and the amino acid sequence of natural human IL-2 is different. The IL-2 mutein was expressed in E. coli and purified by diafiltration and ion exchange chromatography as described in US Pat. No. 4,931,543. IL-2 preparations with Proleukin Provided under the trade name, it is a sterile, white to off-white preservative-free lyophilized powder, containing 1.3mg protein (22MIU) per vial.
[0231] B. Anti-CD20 Antibody
[0232] The anti-CD20 antibody used in this example and the following examples is Rituxan (Rituximab; IDEC-C2...
Embodiment 2
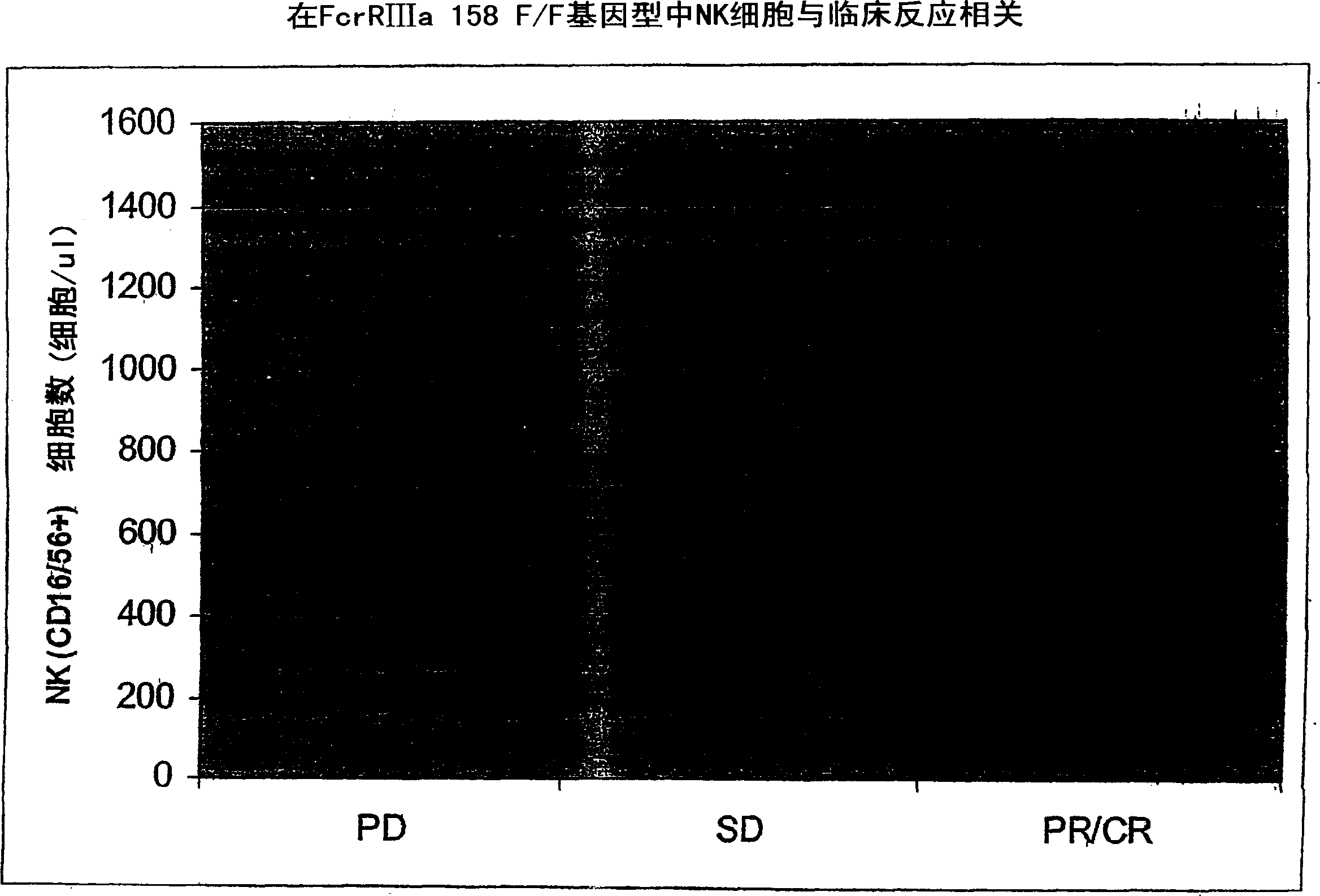
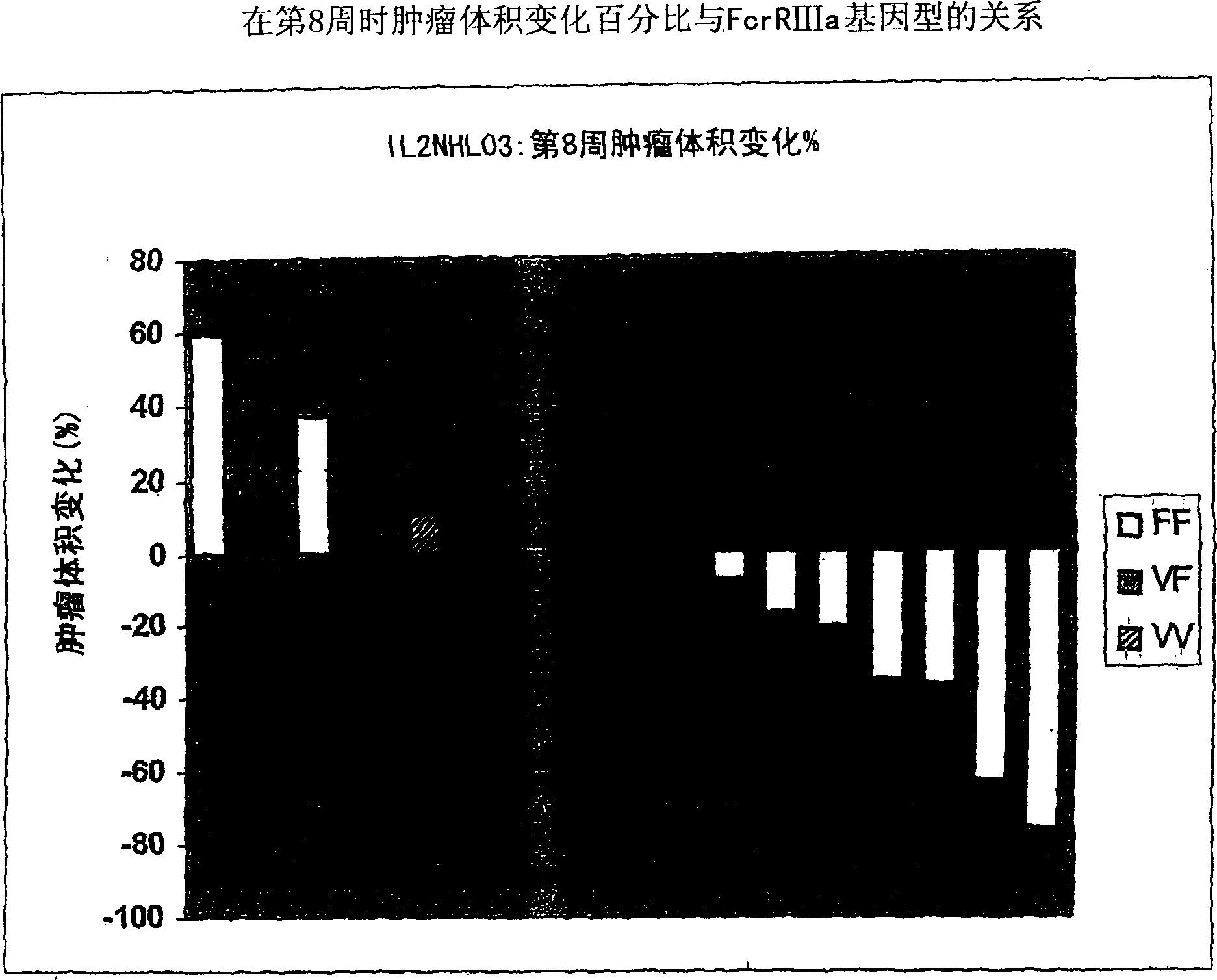
[0247] Example 2: Combination of IL-2-rituximab in a xenograft model of human B-cell non-Hodgkin's lymphoma
[0248] IL-2 was evaluated in two different xenograft models of human B-cell lymphoma (Proleukin ) in combination with rituximab. See, eg, the Namalwa and Daudi xenograft models described by Hudson et al., (1998), Leukemia, 12(12):2029-2033.
[0249] feature
Namalwa
Daudi
CD20 expression
Low
high
disease state
Aggressive
B
tolerance
Responsive
Efficacy of IL-2
efficient
Low curative effect
model duration
Two weeks
6 weeks
[0250] Namalwa or Daudi tumor cells were implanted into mice when the tumor was 100-200mm 3 During the phase, rituximab and / or IL-2 is usually administered 8-12 days after tumor cell implantation.
[0251] The following is a single drug dosage regimen. One group of mice received 0.25 mg / kg of IL-2 subcutaneously (s.c.) p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com