Clonidine, weekly-effect, skin-permeable and control-released plaster
A clonidine transdermal technology, which is applied in the field of clonidine weekly transdermal controlled-release patch, can solve the problems of skin incompatibility, poor adhesiveness, allergies and inflammation easily, and achieve reduced total area and good viscosity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
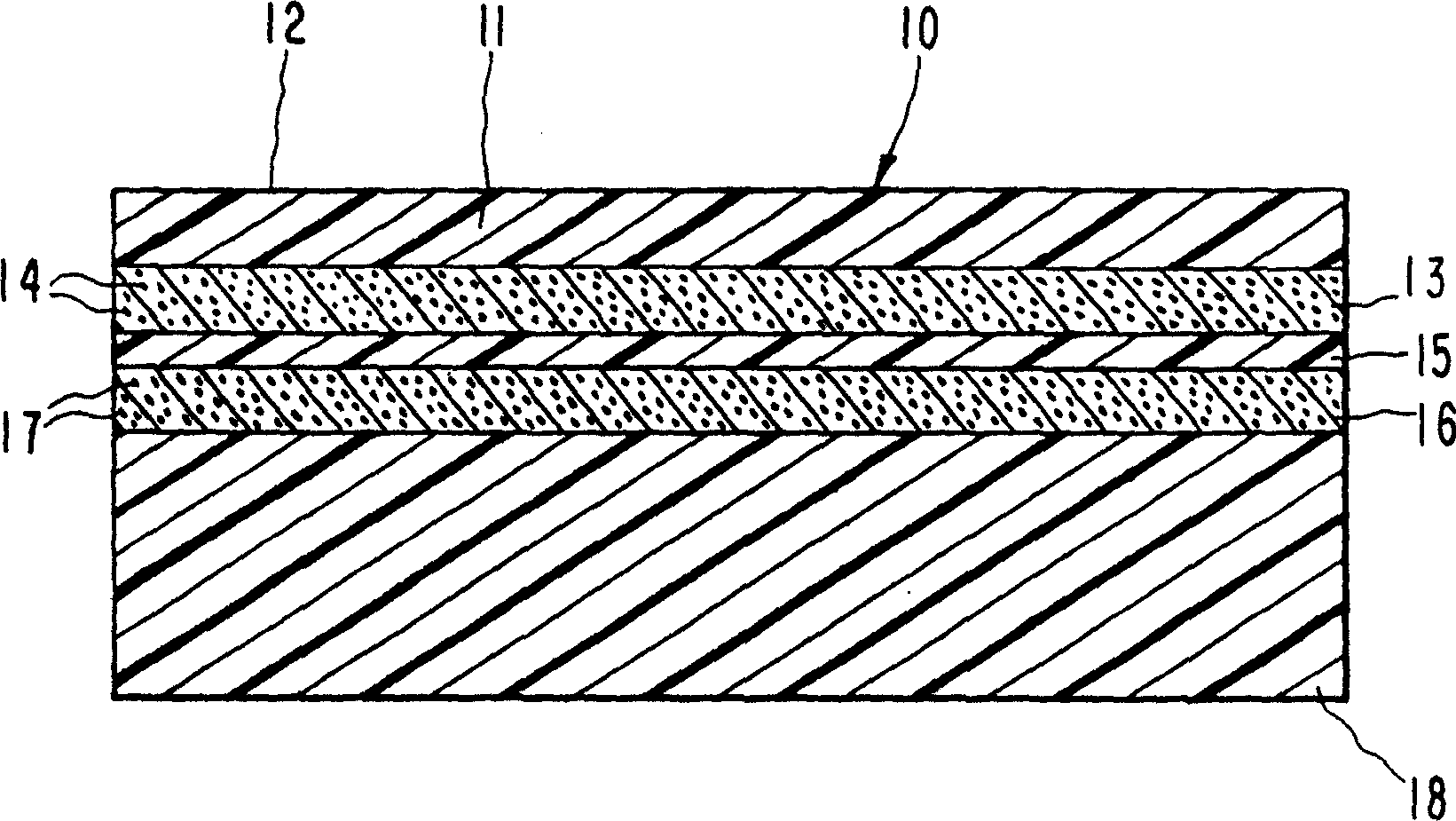
Image
Examples
Embodiment Construction
[0017] 1. Preparation of Acrylate-Ethylene Acetate Copolymer Pressure Sensitive Adhesive
[0018] Monomer components:
[0019] 2-Ethylhexyl Acrylate (2-Ethylhexyl Acrylate) (2-EHA) 75%
[0020] Acrylic Acid (AA) 5%
[0021] Vinyl Acetate (Vac) 20%
[0022] Solvent composition:
[0023] Cyclohexane 100%
[0024] Initiator: BPO or AIBN at 0.4% of the monomer.
[0025] According to the above-mentioned monomer and solvent components, use the traditional solution-type acrylate pressure-sensitive adhesive polymerization process to polymerize into a non-cross-linked pressure-sensitive adhesive solution (the nominal solid content can be calculated as 35%)
[0026] Polymerization process:
[0027] Add 100 parts by mass (wt.%) of the above three mixed monomer components and 80 parts of the above-mentioned solvent into a multi-necked flask equipped with a reflux condenser, a stirrer, a thermometer, a nitrogen inlet tube and a dropping funnel, and heat under stirring And nitrogen g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com