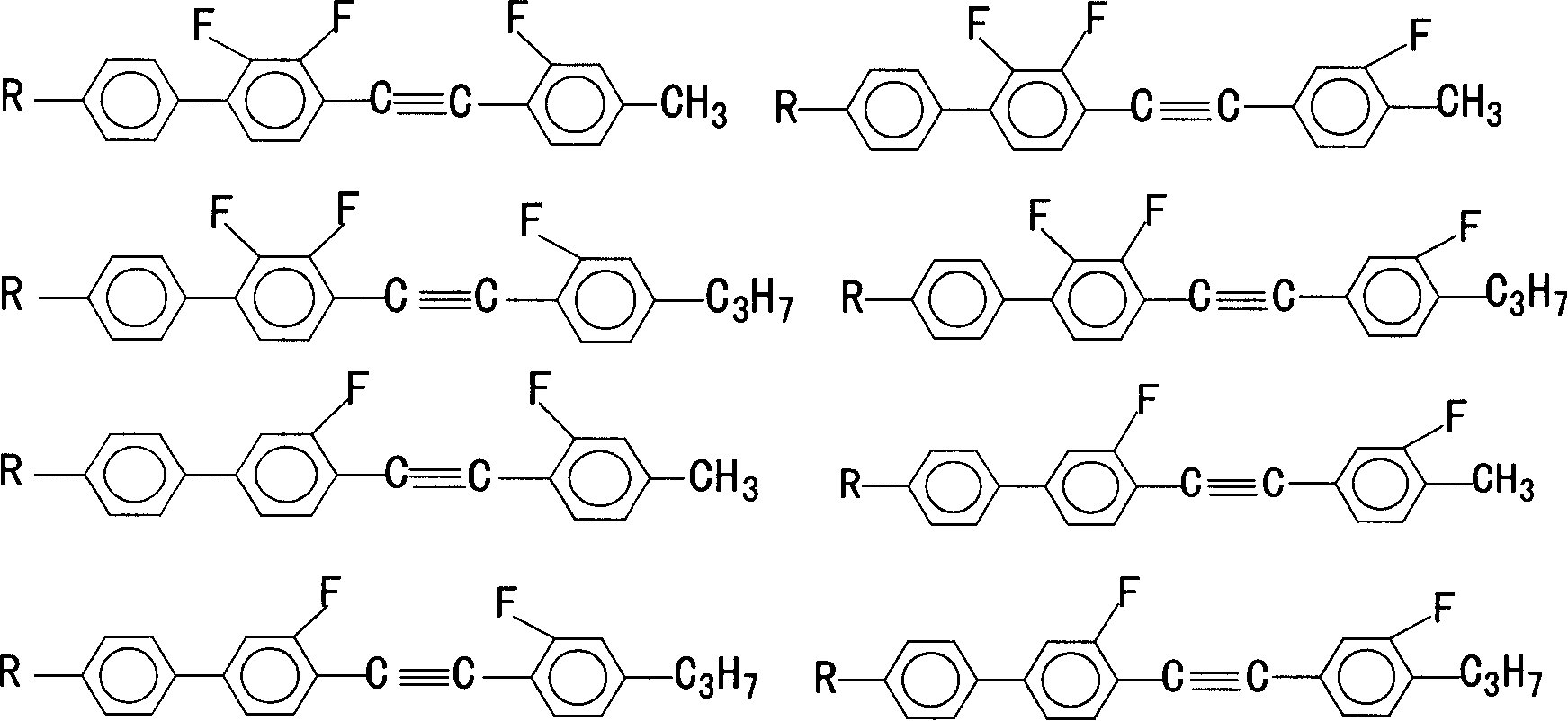
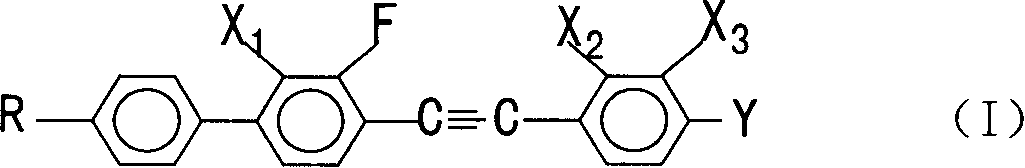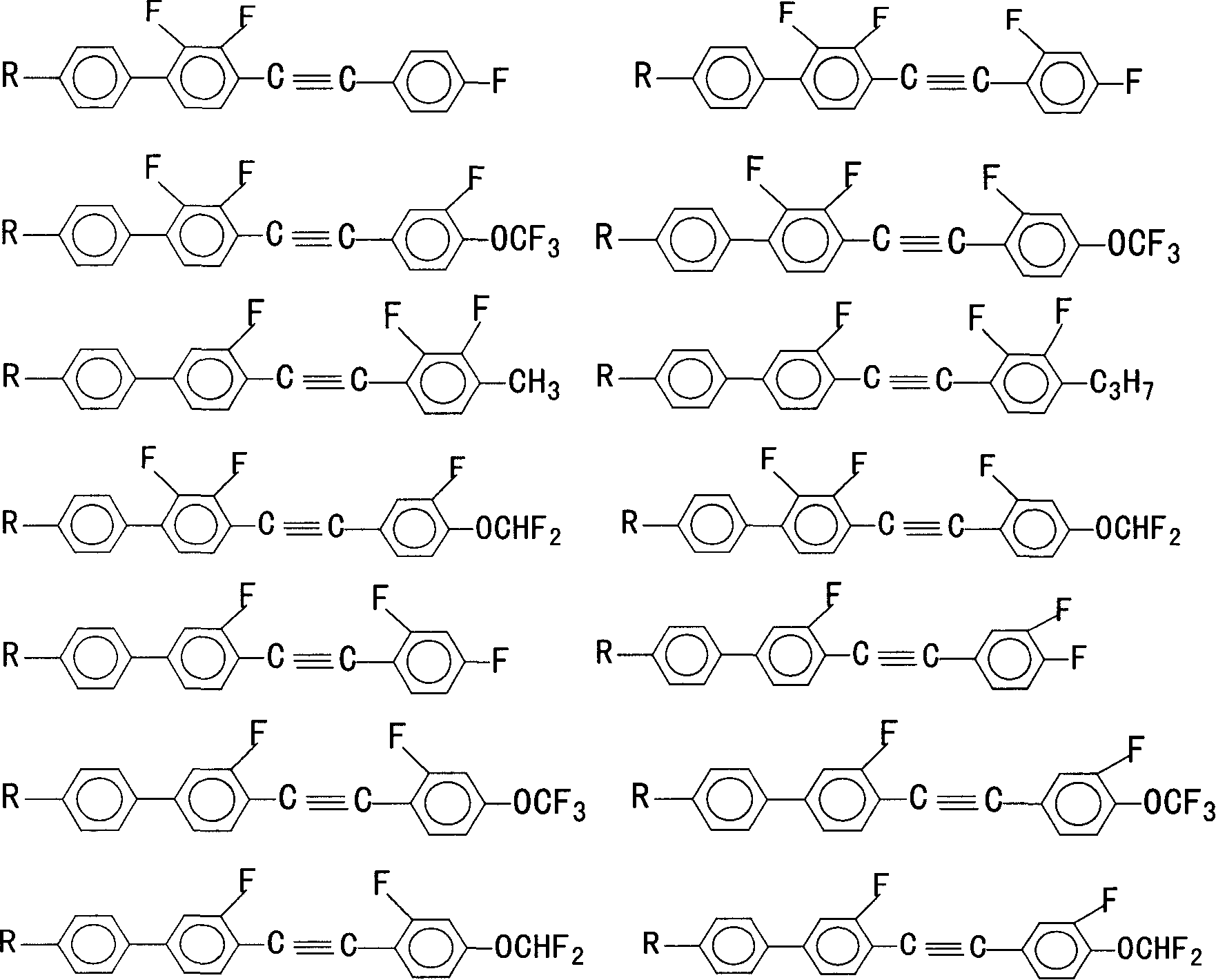Multifluoro substituted diphenyl acetylene derivative, composition containing multifluoro substituted diphenyl acetylene derivatire, its preparation method and use
A technology of diphenylacetylene and derivatives, applied in the field of compounds, can solve problems such as limited compatibility, and achieve the effects of good miscibility, wide nematic phase temperature range, and simple and feasible synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This example is the preparation of 2,3-difluoro-4-(4``-butylphenyl)-2`-fluoro-4`-methyltolanyne (I-1)
[0057]
[0058] Step 1-1: Synthesis of 4`-butyl-2,3-difluorobiphenyl: Add 25.5g (0.12mol) 4-butylbromobenzene, 2,3-difluorophenylboronic acid 15.8 g (0.1mol), 21.2g (0.2mol) of sodium carbonate, 100ml of toluene, 80ml of ethanol, 60ml of water, 0.5g of Pd[0], exhaust the air with nitrogen, heat and reflux for 7 hours, cool down, and pour it into a 1L separatory funnel , separated to remove the water layer, extracted twice with 25ml toluene, combined the organic layers, washed with water until neutral, evaporated toluene to dryness, collected 17.5g of 134~136°C / 3mmHg fraction under reduced pressure, and the gas chromatography purity of the product was ≥99%, and the yield was 71 %.
[0059] Step 1-2: Synthesis of 4`-butyl-2,3-difluorobiphenyl iodide: add 29.6g (0.12mol) 4`-butyl-2,3-difluorobiphenyl into a three-necked flask, Cool 200mlTHF to -80°C, start to drop 0...
Embodiment 2
[0070] This example is the preparation of 2,3-difluoro-4-(4``-butylphenyl)-3`-fluoro-4`-methyltolanyne (I-2)
[0071]
[0072] Preparation Process Example 1, the difference is that the raw material 3-fluoro-4-bromotoluene in step 2-1 is replaced by 2-fluoro-4-bromotoluene to prepare 3-fluoro-4-methylphenylacetylene, Then it was coupled with the product 4'-butyl-2,3-difluoroiodobiphenyl in step 1-2, and purified by recrystallization to obtain the target compound (I-2), with a total yield of 34%.
[0073] The experimental results are as follows:
[0074] (1) Compound phase texture: C56.85°C S m 59.19°C N133.29°C I.
[0075] molecular formula
Characteristic ions (M / Z + ) and abundance (%)
C 25 h 21 f 3
378(M + 、58.97)、335(100)、319(11.38)、167(6.06)
[0076] (3) Elemental analysis:
[0077] Theoretical calculation value: C (79.35%), H (5.59%);
[0078] Experimental values: C (79.33%), H (5.60%).
Embodiment 3
[0080] This embodiment is the preparation (I-3) of 2,3-difluoro-4-(4``-butylphenyl)-2`-fluoro-4`-propyl tolueneacetylene:
[0081]
[0082] The preparation process is the same as in Example 1, except that the raw material 3-fluoro-4-bromotoluene in step 2-1 is changed to 3-fluoro-4-bromopropylbenzene.
[0083] The experimental results are as follows:
[0084] (1) Compound phase texture: C46.57℃ N158.03℃I
[0085] molecular formula
Characteristic ions (M / Z + ) and abundance (%)
C 27 h 25 f 3
406(M + 、100)、377(20.75)、363(88.37)、334(52.12)、167(11.79)
[0086] (3) Elemental analysis:
[0087] Theoretical calculation value: C (79.78%), H (6.20%);
[0088] Experimental values: C (79.77%), H (6.18%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com