Preparation method of activated insulin-like growth factor-II mediated by insulin-like growth factor binding protein-6
A growth factor, insulin-like technology, applied in the fields of pharmacy, biochemistry, molecular biology, biotechnology and pharmacy, biological products, clinical pharmacy, can solve the problem of activity loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
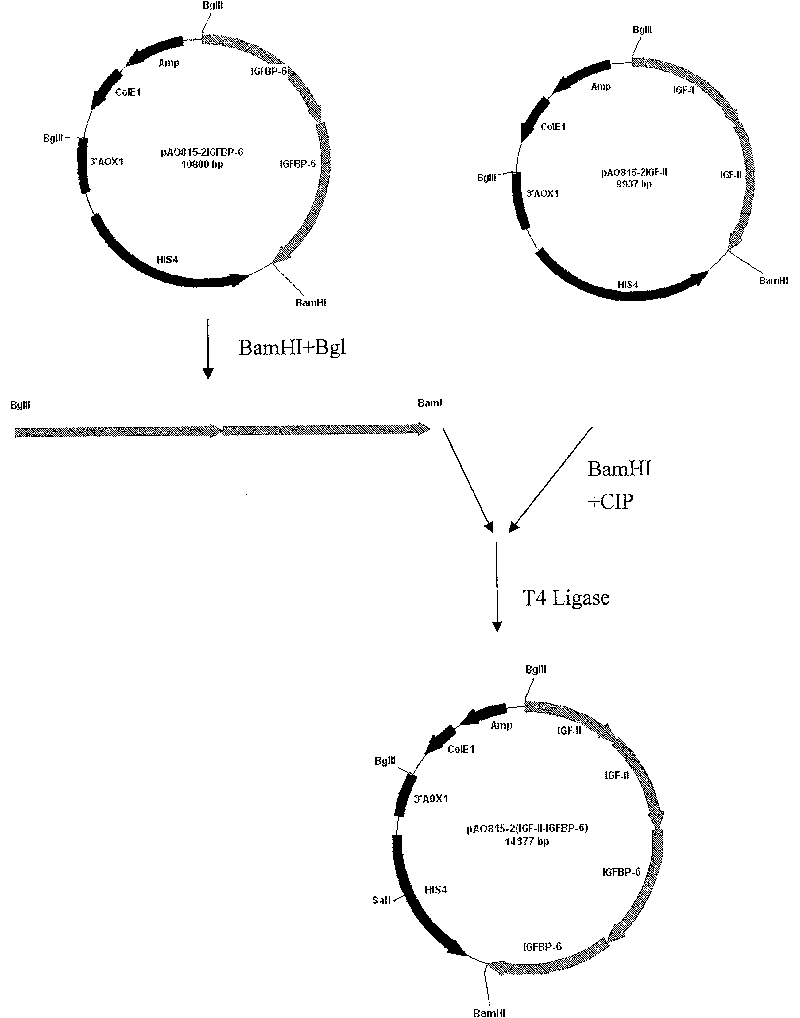
[0070] Example 1. Construction of co-expression vector pAO815-2 (IGF-II-IGFBP-6)
[0071] Utilize the existing expression vector pAO815-2IGF-II and pAO815-2IGFBP-6 to construct the co-expression vector pAO815-2 (IGF-II-IGFBP-6), the method is as follows figure 1 As shown, pAO815-2IGFBP-6 was digested with BglII and BamHI to obtain two copies of the IGFBP-6 expression unit. After recovering the fragment, it was ligated with the expression vector pAO815-2IGF-II that had been linearized by BamHI and dephosphorylated by CIP .Pick ampicillin resistance-positive clones, prepare plasmids, and identify positive clones inserted with the target fragment according to the size of the plasmids, and then use BglII and BamHI double enzyme digestion to identify and screen two copies of IGFBP-6 expression units and connect them in the correct direction The recombinant expression vector pAO815-2(IGF-II-IGFBP-6). Prepare the plasmid, linearize it with SalI, and transform it into Pp strain GS115....
Embodiment 2
[0080] Example 2. Screening of positive co-expression transformants by sticking membrane method
[0081]Inoculate the positive transformants grown on the MD plate onto a new MD plate in sequence, and about 30-40 clones can be inoculated on a plate with a diameter of 9 cm. After culturing at 28-30°C for 3-4 days, the diameter of the clones can reach about 2-3mm. Stick a round sterile cellulose acetate membrane with a diameter of 6 cm on the flat plate to make the colonies adhere to the cellulose acetate membrane. On a freshly prepared BMMY plate, place a piece of sterile nitrocellulose membrane of the same size, put the side of the cellulose acetate membrane with the colony up on the nitrocellulose membrane, so that the two membranes overlap, Try to eliminate air bubbles between the two membranes and the plates. Induce the expression for 24 hours at 28-30°C, and remove the nitrocellulose membrane for antibody hybridization. For each experiment, two identical membranes should...
Embodiment 3
[0083] Embodiment three, shaking flask expression
[0084] After SalI linearized pAO815-2 (IGF-II-IGFBP-6) was transformed into GS115, the HIS4 gene on the vector and the his4 gene on the yeast chromosome underwent homologous recombination and integrated into the yeast chromosome without affecting the yeast AOX1 promoter pair The utilization of methanol, the obtained positive transformants should all be Mut + Phenotype. Increase the volume of culture when inducing expression. Scrape the positive clones on the MD plate, inoculate them in 25ml YPD liquid medium, shake and culture at 28~30℃, 200~230rpm for 20h, then transfer an appropriate amount of bacteria solution to 100ml BMG and continue culturing overnight to make the OD 600 ≈10, centrifuge at room temperature at 1500×g, collect the bacteria, resuspend in 500ml BMMY, induce culture with 1% methanol for 72 hours, collect the supernatant by centrifugation at 8000×g at 4°C, store at 4°C for short-term storage, if long-term s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com