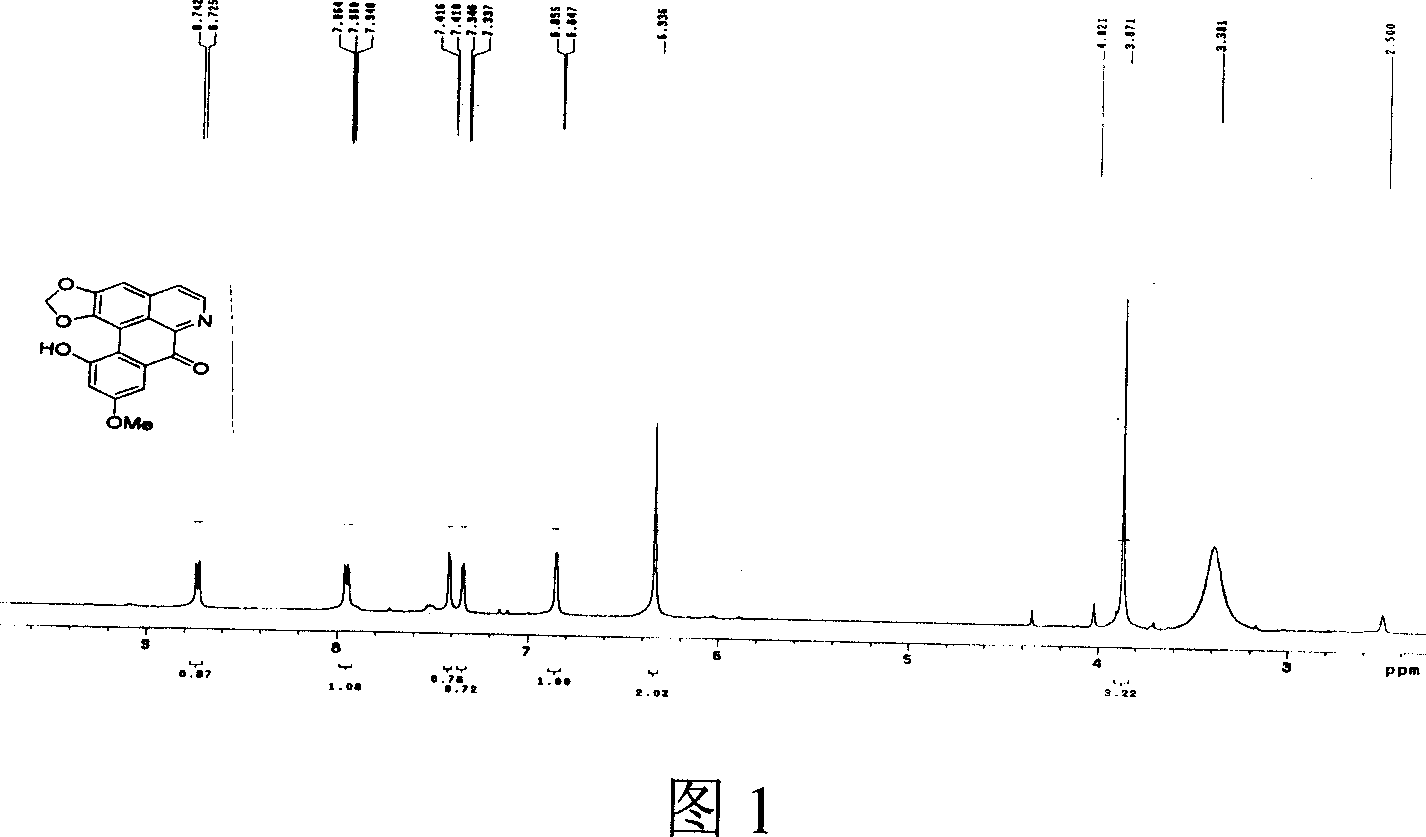
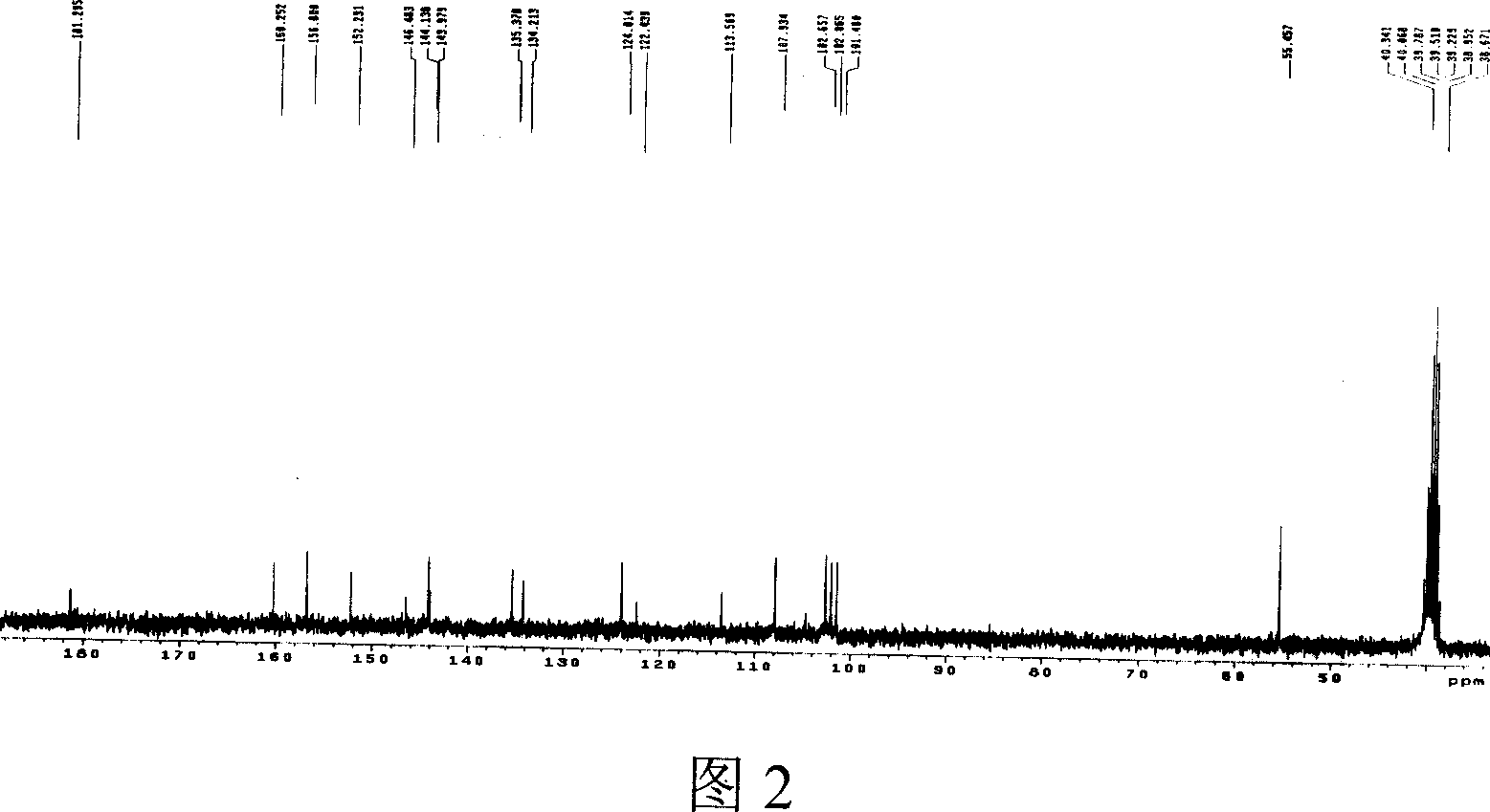
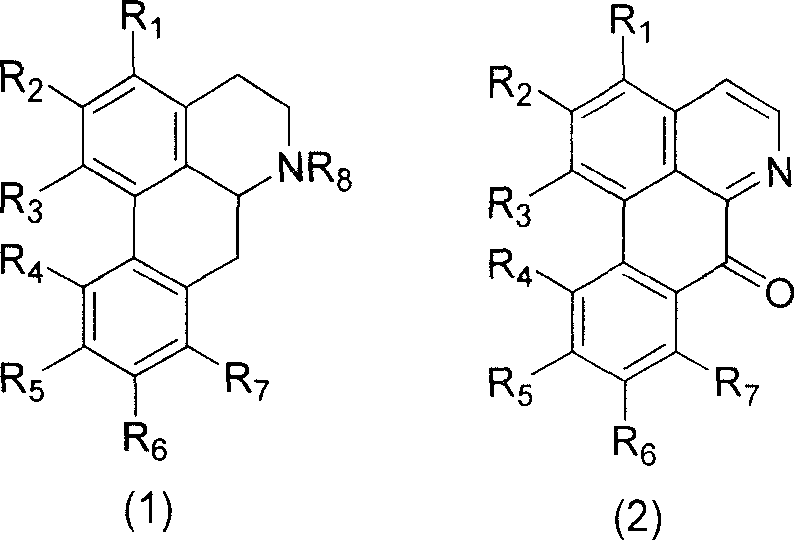
Aporphine and use of oxidized aporphine alkaloid
A technology for oxidizing aporphi and alkaloids, which is applied in the fields of anti-inflammation and immunosuppression of aporphi and oxidized aporphim alkaloids, and can solve the problems of in-depth research on the composition and content of total alkaloid monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1000 grams of Zhanshanfeng cane or root (collected from Jiangxi), heat extraction 3 times with 2% sulfuric acid aqueous solution (3800 milliliters for the first time, 3000 milliliters for the second time, 3000 milliliters for the third time), 3 hours each time, filter Then apply D101 macroporous resin, wash with water first, then wash with 20%, discard, elute with 50% ethanol aqueous solution, and concentrate under reduced pressure to obtain 6.4 grams of total alkali sulfate sample. The content of total alkaloid sulfate (calycinine sulfate as reference substance, weight ratio) measured by ultraviolet spectrophotometry is 53.4%. The contents of calycinine sulfate and xylopine sulfate measured by HPLC were 6.4% and 4.8%, respectively.
Embodiment 2
[0070] 1000 grams of Zhanshanfeng cane or root (collected from Jiangxi), heat extraction 3 times with 2% sulfuric acid aqueous solution (3800 milliliters for the first time, 3000 milliliters for the second time, 3000 milliliters for the third time), 3 hours each time, filter Finally, adjust the pH to 8-9 with ammonia water, then adjust to 30% ethanol solution with ethanol, apply D101 macroporous resin, elute with 30% and 90% ethanol aqueous solution, and concentrate 90% of the eluted fraction under reduced pressure to obtain Total alkali sample 4.9 grams. The total alkaloid content (with calycinine as reference substance, weight ratio) measured by ultraviolet spectrophotometry is 59.8%. The contents of calycinine and xylopine measured by HPLC were 5.7% and 5.3%, respectively.
Embodiment 3
[0072] 1000 grams of Zhanshanfeng cane or root (collected from Jiangxi), heat extraction 3 times with 80% ethanol aqueous solution (3800 milliliters for the first time, 3000 milliliters for the second time, 3000 milliliters for the third time), 3 hours each time, filter After concentrating to the extractum, the extractum was kneaded and dissolved with 200 ml of 2% sulfuric acid, filtered and cleared onto D101 macroporous resin, washed with water first, then eluted with 20%, 50% ethanol aqueous solution, and washed with 50% ethanol aqueous solution. The dedistillation fraction was concentrated under reduced pressure to obtain 6.0 g of total alkali salt sample. The content of total alkaloid sulfate (calycinine sulfate as reference substance, weight ratio) measured by ultraviolet spectrophotometry is 51.8%. The contents of calycinine sulfate and xylopine sulfate measured by HPLC were 6.0% and 5.1%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com