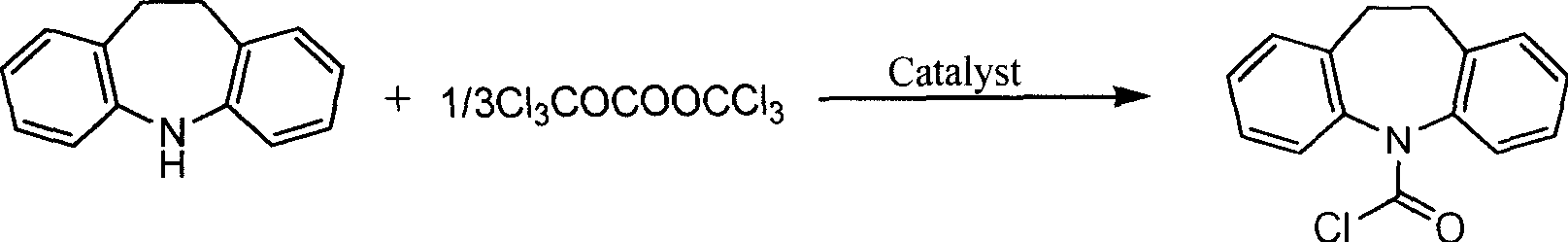Chemically synthetic method for N-chloroformyl imino dibenzyl
A technology of chloroformyl imidodibenzyl and iminodibenzyl: double, which is applied in the field of chemical synthesis of N-chloroformyl imidodibenzyl, can solve the problem that the phosgene reaction cannot be completely quantified and the reaction conditions are harsh , safety hazards and other issues, to achieve the effect of solving the problem of tail gas absorption, high yield and purity, and avoiding difficult storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The amount ratio of the feed material is iminodibenzyl: bis(trichloromethyl)carbonate: organic amine catalyst is 1.0: 0.45: 0.05, wherein the organic amine catalyst is pyridine, the organic solvent is tetrahydrofuran, and its consumption is imino 8 times the mass of dibenzyl.
[0021] In a 250mL three-necked flask with mechanical stirring, reflux condenser (with tail gas absorption device), and a thermometer, add 19.26g (100mmol) of iminodibenzyl, bis(trichloromethyl)carbonate 13.35g (45mmol) , pyridine 0.395g (5mmol) and 154g tetrahydrofuran, open mechanical stirring, treat that solid dissolves completely, be heated to tetrahydrofuran reflux temperature, react 12 hours, monitor reaction process with HPLC during the reaction (mobile phase: acetonitrile: water: tetrahydrofuran=40:50 : 10, C18 silica gel column, flow rate 1.0mL / min). After the reaction was completed, part of the solvent was evaporated under normal pressure to obtain a saturated solution, which was cooled...
Embodiment 2
[0023] The amount ratio of the feed material is iminodibenzyl: bis(trichloromethyl)carbonate: organic amine catalyst is 1.0: 0.40: 0.05, wherein the organic amine catalyst is pyridine, the organic solvent is tetrahydrofuran, and its consumption is imino 8 times the mass of dibenzyl.
[0024] The reaction temperature was THF reflux temperature, and the reaction time was 15 hours. Other operations were the same as in Example 1 to obtain 25.1 g of N-chloroformylimidodibenzyl, with a product yield of 97.2% and a purity of 99.2%.
Embodiment 3
[0026] The amount ratio of feed material is iminodibenzyl: two (trichloromethyl) carbonate: organic amine catalyst is 1.0: 0.35: 0.05, wherein organic amine catalyst is pyridine, organic solvent is tetrahydrofuran, and its consumption is imino 8 times the mass of dibenzyl.
[0027] The reaction temperature was reflux temperature, and the reaction time was 15 hours. Other operations were the same as in Example 1 to obtain 24.8 g of N-chloroformyliminodibenzyl, with a product yield of 96.5% and a purity of 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com