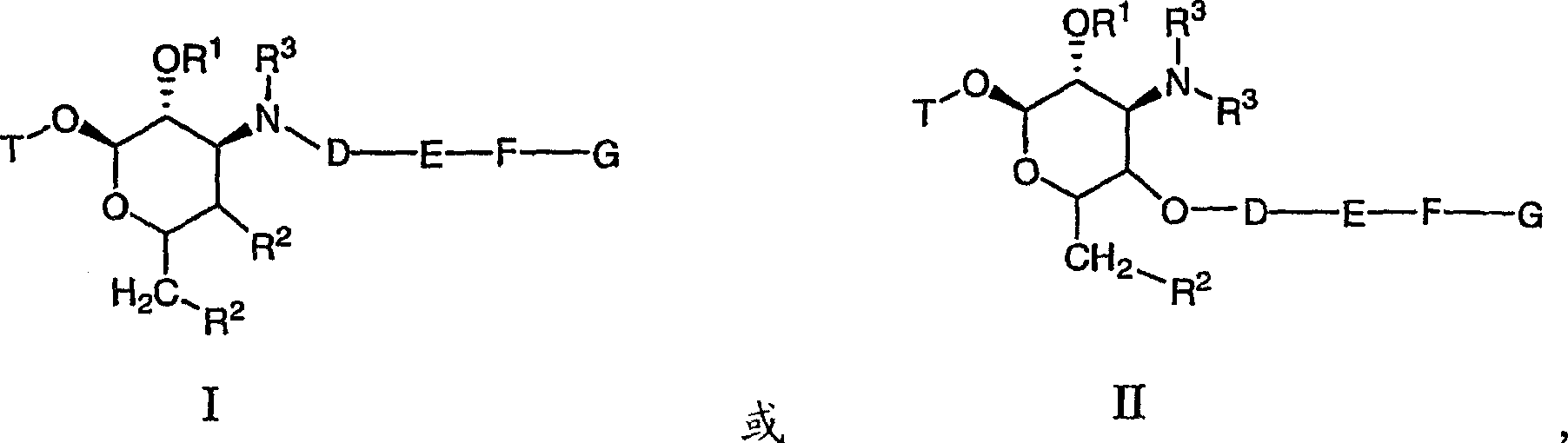
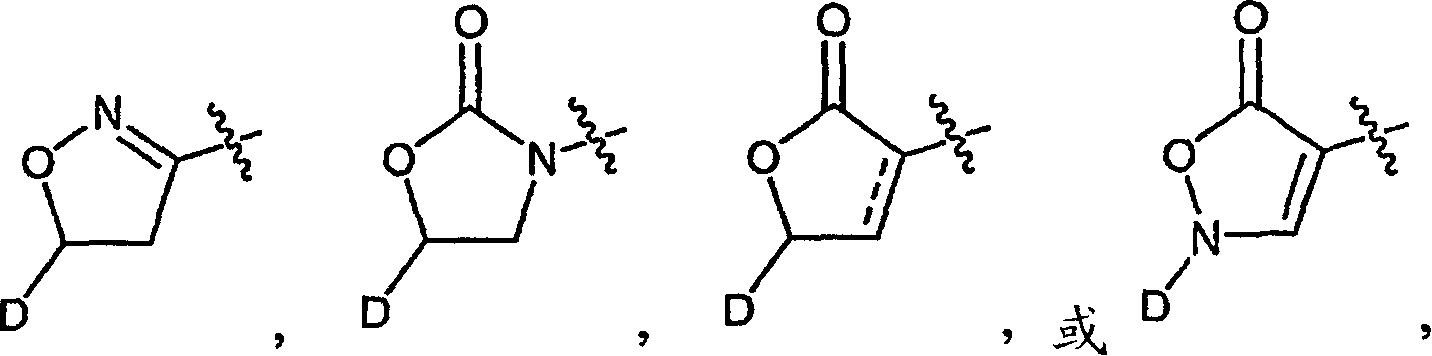
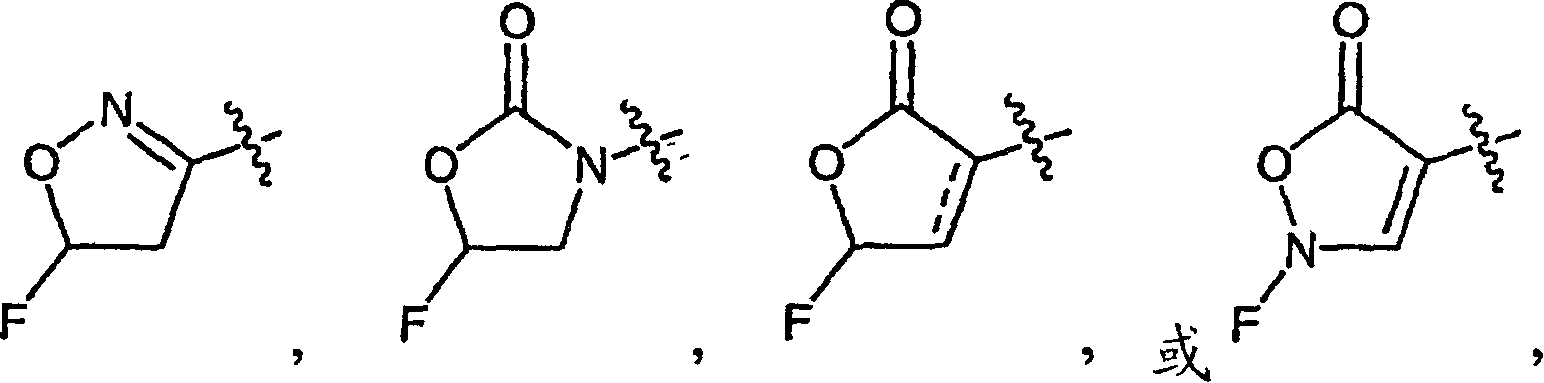
Macrocyclic compounds and methods of making and using the same
A technology of compounds and oxides, applied in the field of peristaltic accelerators and a family of macrocyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0603] Example 1 - Synthesis of Compounds 101-280
[0604] Schemes 100 and 101 below describe the synthesis of compounds 101-280. 3'-N-desmethylazithromycin 2 is selectively generated by demethylation of azithromycin 1. Amine 2 was selectively alkylated with tosylate 11, 12, 13 to generate alkynes 3, 4, 5, respectively. As shown in Scheme 101, alkynes 3, 4 or 5 and azides 14a-14gm selectively provide triazoles 101-280 in the presence of cuprous iodide.
[0605] Scheme 100: Synthesis of alkynes 3, 4 and 5.
[0606]
[0607] Synthesis of 3'-N-desmethylazithromycin 2
[0608] Azithromycin 1 (0.80 g, 1.02 mmol) and sodium acetate (0.712 g, 8.06 mmol) were dissolved in 80% aqueous methanol (25 mL). The solution was heated to 50°C, then iodine (0.272 g, 1.07 mmol) was added in three portions over three minutes. The pH of the reaction was maintained between 8-9 by adding 1N sodium hydroxide (1 ml) at intervals of 10-45. The solution became a colorless liquid within 45 minute...
Embodiment 2
[0641] Example 2 - Synthesis of Compounds 301-357
[0642] Below; Schemes 103 and 104 are the synthesis of compounds 301-357. 3'-N-desmethylerythromycin A20 is selectively generated by demethylation of erythromycin A. Likewise, demethyl clarithromycin produces 3'-N-desmethyl clarithromycin21. Selective N-alkylation of amines 20 and 21 with propargyl bromide or with tosylate 11 or 12 yielded alkynes 23, 24, 25, 26, 27, or 28, respectively. As shown in Scheme 2, alkynes 23-28 and azides 14a-14eb selectively provide triazoles 301-357 in the presence of cuprous iodide.
[0643]
[0644] Synthesis of 3'-N-desmethylerythromycin A20
[0645] Compound 20 was prepared from erythromycin using the method described in U.S. Patent No. 3,725,385.
[0646] Synthesis of 3'-N-desmethyl clarithromycin 21
[0647] In clarithromycin (1.00 g, 1.3 mmol) and NaOAc·H 2 O (0.885 g, 6.5 mmol) was added to the mixture of MeOH-H 2 O (20ml, 4:1), the mixture was heated to 55-60°C. Iodine (0.330...
Embodiment 3
[0668] Example 3 - Synthesis of Compounds 401-417
[0669] Compounds 401-417 shown in Table 4 were derived from telithromycin by a method similar to Tables 2 and 3 above. Telithromycin, like azithromycin, erythromycin and clarithromycin as described above, is selectively demethylated and then alkylated with tosylate. As a result, alkynes were added to the corresponding triazoles via the same copper cycloaddition catalysis [3+2] as described above for azide 14.
[0670] Synthesis of 3'-N-desmethyltelithromycin 30
[0671] To a solution of telithromycin 29 (3.0 g, 3.60 mmol) in anhydrous acetonitrile was added N-iodosuccinimide (NIS) (0.98 g, 4.32 mmol) in two portions with argon at 0 °C Air for 30 minutes. The mixture was warmed to room temperature and stirred overnight. Join CH 2 CL 2 (250 ml) and 5% Na 2 S 2 o 3 (80 mL) until the two layers were separated. Organic layer with 5% Na 2 S 2 o 3 (1 x 80 mL) extracted with NH 4 CL (1×80 ml) diluted with Na 2 SO 4 dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com