A cell mediated immunity vaccine and preparation method thereof
A technology of cellular immunity and vaccines, applied in pharmaceutical formulas, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve problems such as uncontrollable diseases and lagging vaccines, and achieve significant curative effect, low cost, and fast results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, the preparation of pullorumosis vaccine and its detection
[0028] 1. Prepare the vaccine
[0029] 1. Acquisition and purification of mononuclear cells
[0030] 1) Isolation of mononuclear cells
[0031] Isolate mononuclear cells from chickens. The specific method is: sterilize the blood collection site under the chicken wings, collect blood from the vein, add heparin sodium solution with a volume percentage concentration of 5% to anticoagulate, and then use lymphocyte separation solution from the anticoagulant blood (purchased from Cedarlane Company) to obtain mononuclear cells by density gradient centrifugation.
[0032] 2) Purification of mononuclear cells
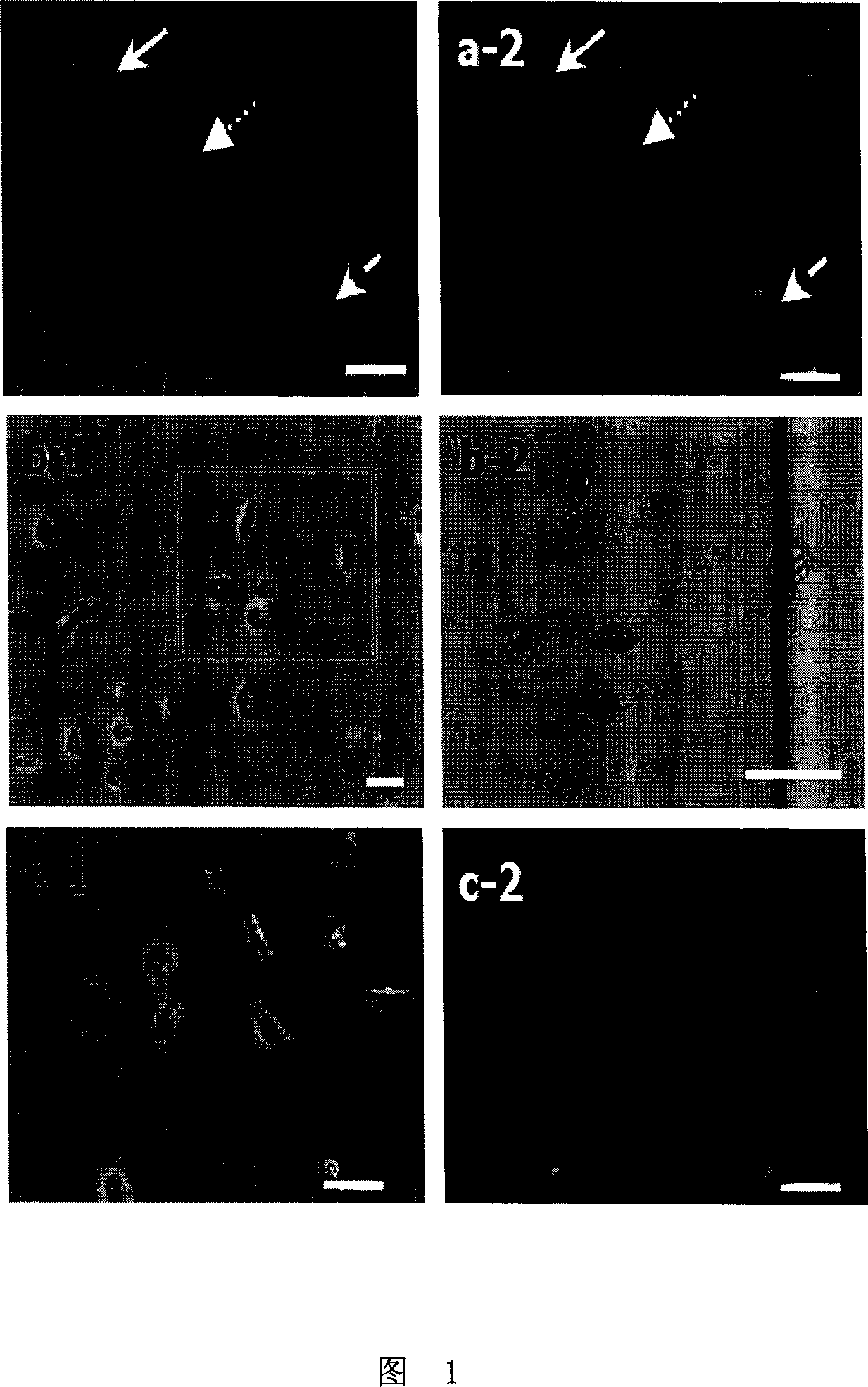
[0033] Mononuclear cells were cultured in complete RPMI1640 medium at 37°C, 5% CO 2 Cultivate in a constant humidity incubator for 8-24 hours. After the cells adhere to the wall, remove the suspended cells, and carry out acridine orange (AO) staining, Giemsa staining and CD14 indirect immunofluo...
Embodiment 2
[0044] Embodiment 2, preparation and detection of porcine erysipelas vaccine
[0045] 1. Prepare the vaccine
[0046] 1. Acquisition and purification of mononuclear cells
[0047] 1) Isolation of mononuclear cells
[0048] The specific method for isolating mononuclear cells from pigs is as follows: sterilize the blood collection site of the anterior vena cava of pigs, collect blood from the vein, add sodium citrate solution with a volume percentage concentration of 5% to anticoagulate blood, and then use lymphocytes from the anticoagulant blood Mononuclear cells were obtained from the separation solution by density gradient centrifugation.
[0049] 2) Purification of mononuclear cells
[0050] The mononuclear cells were cultured with RPMI1640 complete culture medium in a 37°C incubator for 24-48 hours. After the cells adhered to the wall, the suspended cells were removed, and the adherent cells were stained with acridine orange (AO), Giemsa staining and CD14 The results of...
Embodiment 3
[0061] Embodiment 3, preparation and detection of bovine tuberculosis vaccine
[0062] 1. Prepare the vaccine
[0063] 1. Acquisition and purification of mononuclear cells
[0064] 1) Isolation of mononuclear cells
[0065] Mononuclear cells are isolated from cattle. The specific method is: sterilize the human venous blood collection site, collect blood from the vein, add sodium citrate solution with a volume concentration of 5% to anticoagulate the blood, and then use the lymphocyte separation fluid to pass through the density of the anticoagulated blood. Mononuclear cells were isolated by gradient centrifugation.
[0066] 2) Purification of mononuclear cells
[0067] Culture mononuclear cells with TCM199 complete culture medium in a 37°C incubator for 24-72 hours. After the cells adhere to the wall, remove the suspended cells, and perform acridine orange (AO) staining, Giemsa staining and CD14 staining on the adherent cells. The results of indirect immunofluorescence sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com