Preparation method for pro-his cyclic dipeptide
A technology of cyclic dipeptide and protease group, which is applied in the field of preparation of peptide drugs, can solve the problems of low yield and few synthesis methods of proteocyclic dipeptide, and achieve simple post-processing, considerable economic use value and low pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
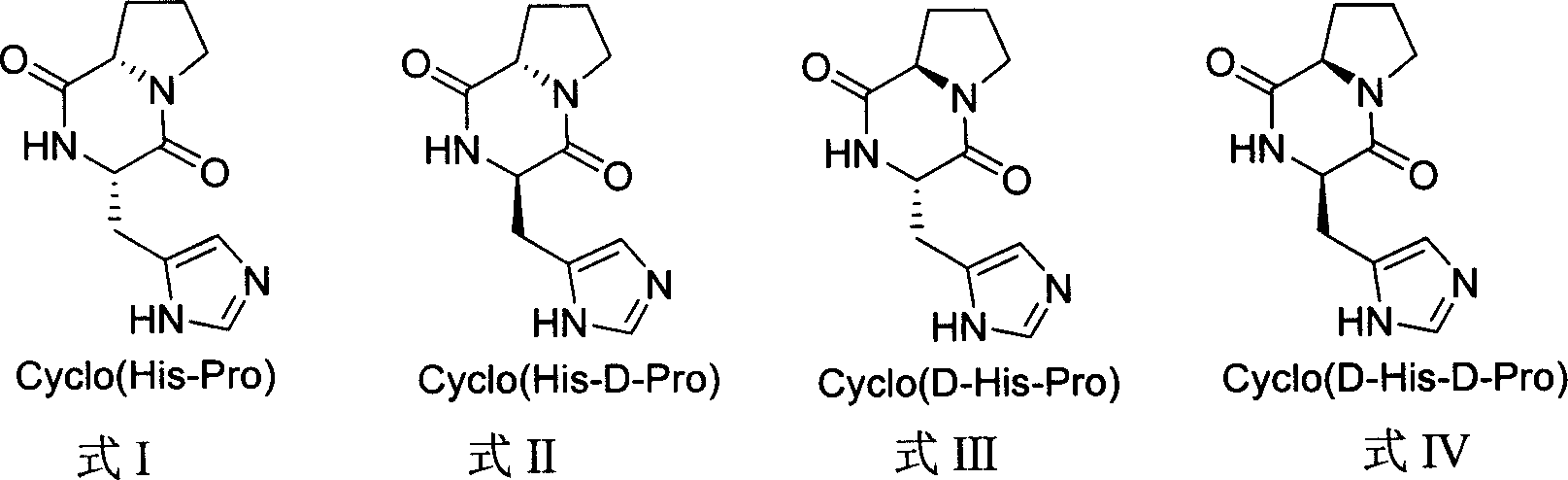
[0028] Embodiment 1, L-L type proline group cyclic dipeptide
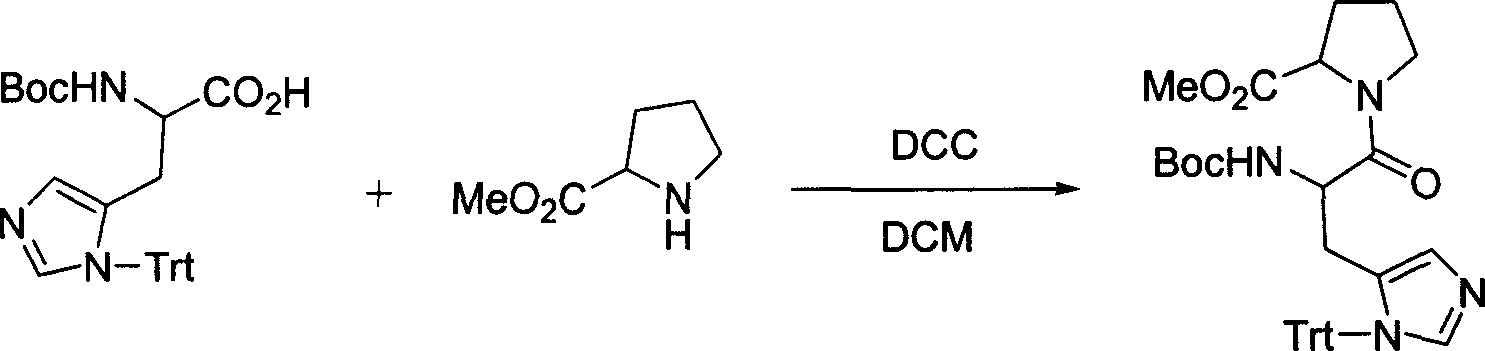
[0029] 1. Add 450 mg of L-form proline methyl ester (Pro-OMe) and 744 mg of Boc-protected L-form histidine (Boc-His-OH) dropwise to 10 ml containing 1150 mg of dicyclohexyl carbon di The imine (DCC) in DCM was stirred at room temperature for 20 hours. After filtration, the reaction solution was concentrated under reduced pressure and separated by silica gel column chromatography to obtain 210 mg of protected promethazine dipeptide methyl ester (Boc-His-Pro-OMe), with a yield of 20.5%. electrospray mass spectrometry (ESI-MS) and nuclear magnetic resonance ( 1 H NMR 13 C NMR) measurement, proves that the obtained product is correct.
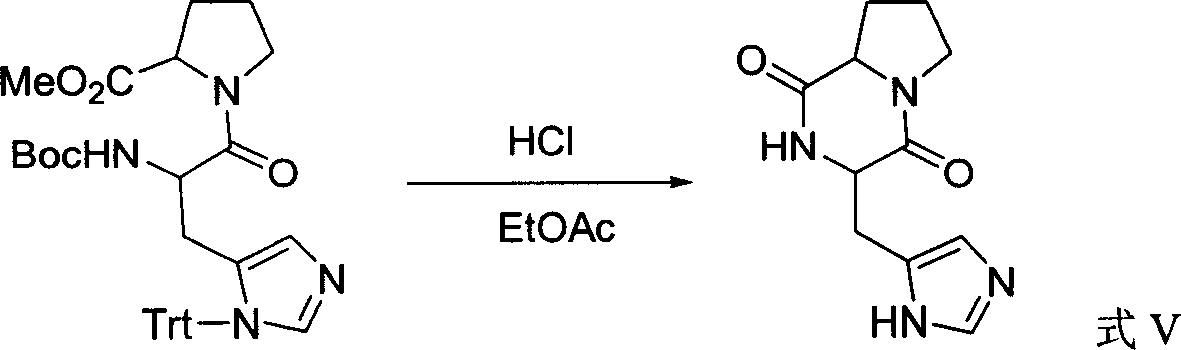
[0030] 2. Dissolve 150 mg of the obtained protected dipeptide methyl ester in 5 ml of ethyl acetate, add 2 ml of trifluoroacetic acid, and stir for 1 hour. The reaction solution was concentrated under reduced pressure to obtain 119 mg of trifluoroacetic acid salt of dipeptide methyl...
Embodiment 2
[0032] Embodiment 2, L-L type proline group cyclic dipeptide
[0033] 1. 450 mg of L-type proline methyl ester (Pro-OMe) and 744 mg of Boc-protected L-type histidine (Boc-His-OH), 320 mg of 4-dimethylaminopyridine (DMAP) Add to 15 ml of dichloromethane (DCM) and stir to dissolve at room temperature, then add dropwise 10 ml of DCM solution containing 1150 mg of dicyclohexylcarbodiimide (DCC), and stir at room temperature for 18 hours. After filtration, the reaction solution was concentrated under reduced pressure and separated by silica gel column chromatography to obtain 270 mg of protected promethazine dipeptide methyl ester (Boc-His-Pro-OMe) with a yield of 26.3%. electrospray mass spectrometry (ESI-MS) and nuclear magnetic resonance ( 1 H NMR 13 C NMR) measurement, proves that the obtained product is correct.
[0034] 2. Dissolve 150 mg of the obtained protected dipeptide methyl ester in 5 ml of ethyl acetate, add 2 ml of trifluoroacetic acid, and stir for 1 hour. The r...
Embodiment 3
[0036] Embodiment 3, L-L type proline group cyclic dipeptide
[0037] 1. 450 mg of L-type proline methyl ester (Pro-OMe) and 744 mg of Boc-protected L-type histidine (Boc-His-OH), 440 mg of 1-hydroxybenzotriazole (HOBt ) was added to 20 ml of dichloromethane (DCM) and stirred at room temperature to dissolve, then 10 ml of DCM solution containing 1150 mg of dicyclohexylcarbodiimide (DCC) was added dropwise, and stirred at room temperature for 4 hours. After filtration, the reaction solution was concentrated under reduced pressure and separated by silica gel column chromatography to obtain 550 mg of protected promethazine dipeptide methyl ester (Boc-His-Pro-OMe), with a yield of 52.3%. electrospray mass spectrometry (ESI-MS) and nuclear magnetic resonance ( 1 H NMR 13 C NMR) measurement, proves that the obtained product is correct.
[0038]2. Dissolve 150 mg of the obtained protected dipeptide methyl ester in 5 ml of ethyl acetate, add 2 ml of trifluoroacetic acid, and stir f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com