Improved olefin plant recovery system employing a combination of catalytic distillation and fixed bed catalytic steps
A catalytic distillation tower, fixed bed reactor technology, applied in cracking gas effluent, olefin production, catalytic distillation field, can solve difficult industrial scale, limited methods of catalyst deactivation, hydrogen flow changes of alkynes and dienes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
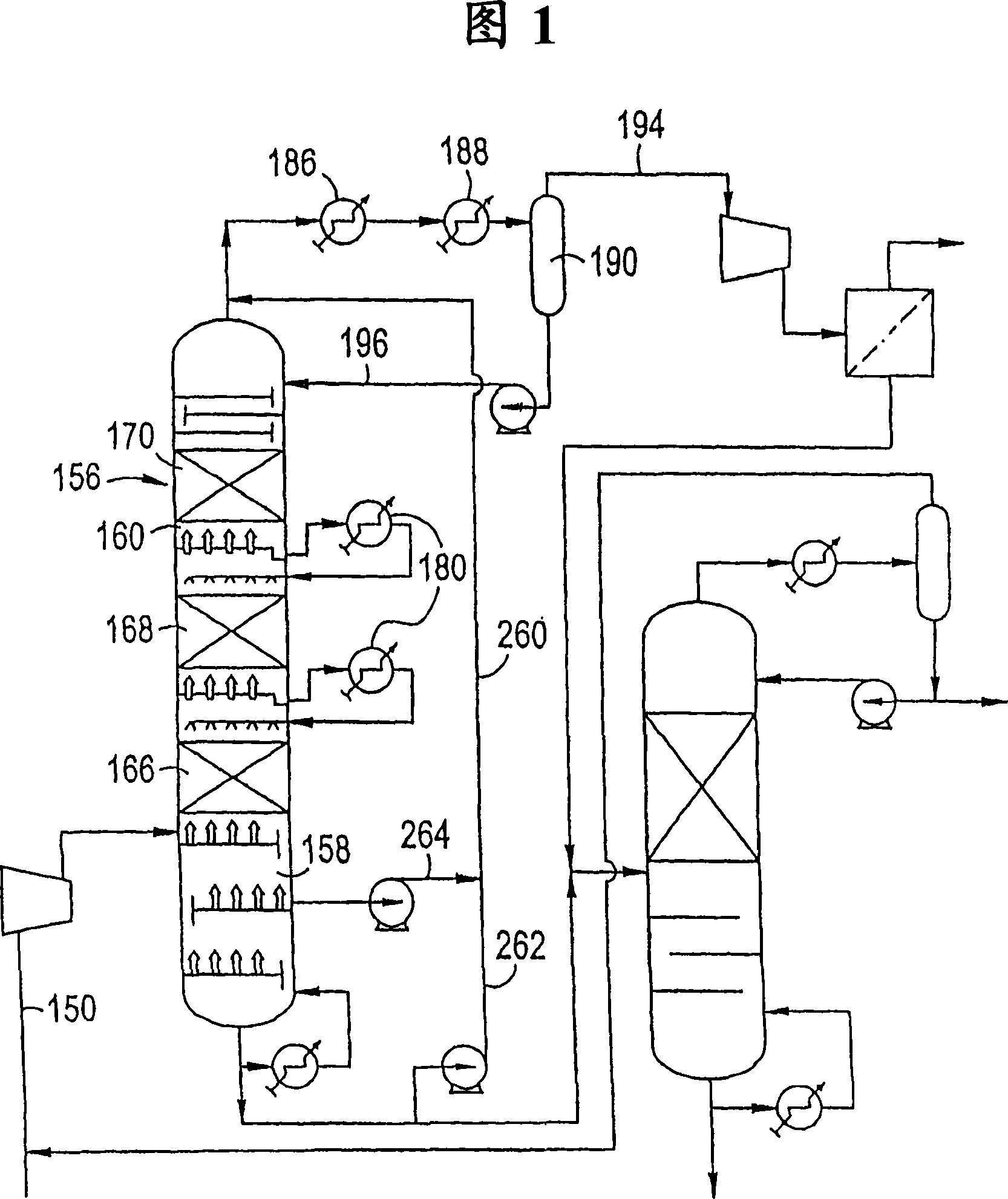
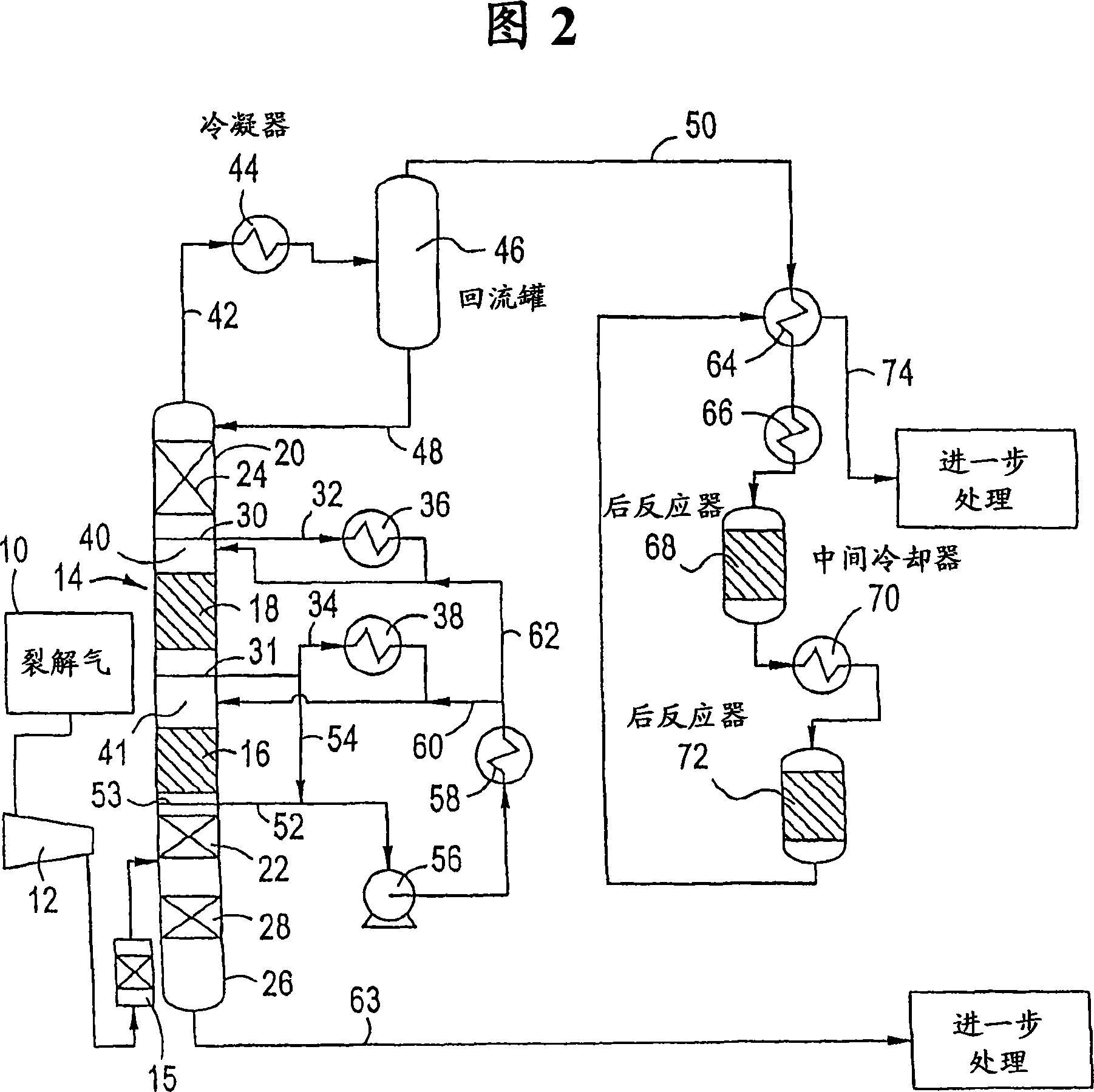
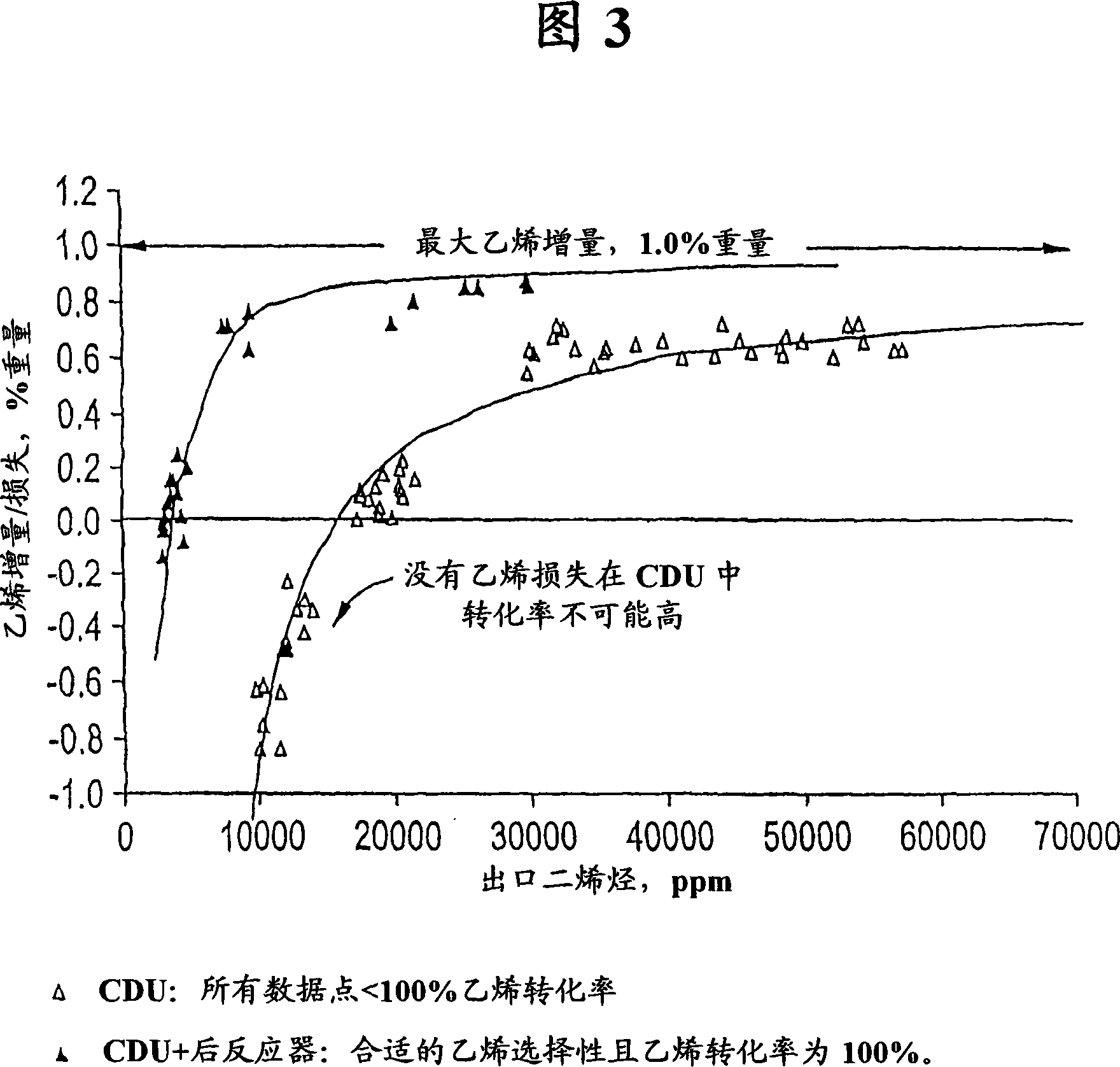
[0064] This example represents the prior art outlined in prior US Patent No. 5,679,241 (Fig. 1), based on a one-step catalytic distillation column operated at a reflux ratio of 4.4. Typical pre-alkyne hydrogenation catalysts contain less than 2000 ppm palladium, operated at a pressure of 195 psig and an average catalyst temperature of 230°F, C 2 Alkyne conversion reached 84% with 0% ethylene loss / gain. At the reactor outlet C 2Alkynes are 370 ppm and dienes and alkynes together are 19070 ppm. The overall alkyne / diene conversion was 79.5%. This example represents a single column run with no ethylene loss. It can be seen that: C 2 Large excess of alkynes. This would result in an off-spec ethylene product.
Embodiment 2
[0066] This example also represents the prior art, based on the single catalytic distillation column of Example 1 (using a higher catalyst temperature and a slightly lower reflux ratio of 4.1). The hydrogenation intensity of a single column can be increased to achieve low C 2 Alkyne levels. This can be accomplished by increasing the temperature or increasing the activity of the catalyst. Higher temperature operation will reduce the alkyne content, so as to obtain qualified ethylene products. Compared to Example 1, the conversion of all dienes and alkynes is higher in this case, however there is also a loss of 0.6% of ethylene. At the reactor outlet, C 2 Alkynes are 240 ppm and dienes and alkynes together are 12340 ppm. The overall alkyne / diene conversion was 86.7%. It can be seen that: C 2 Increased conversion of alkynes leads to increased loss of ethylene. This is uneconomical. In addition, ethylene products still cannot meet the specification limit of 1-2ppm.
Embodiment 3
[0068] This example represents the prior art and is based on the single catalytic distillation column of Example 1 (higher carbon monoxide level in the feed). Carbon monoxide is a catalyst poison, so the conversion of dienes and alkynes is significantly reduced. When the CO level in the feed is 0.1 mole %, the C in the product 2 460 ppm for alkynes and 33860 ppm for dienes and alkynes combined. The CO-induced reduction in catalyst activity resulted in loss of catalyst yield (0.12–0.09 lbmol / hr-ft catalyst structure) and lower overall alkyne / diene conversion (63.6% versus 79.5% in the baseline case).
[0069] The measure taken against the reduction in activity is to increase the temperature of the catalyst in the catalytic distillation column. This would require a pressure increase beyond what the operating unit would actually operate at. The options to compensate for the increase in CO are therefore limited with respect to the prior art.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com