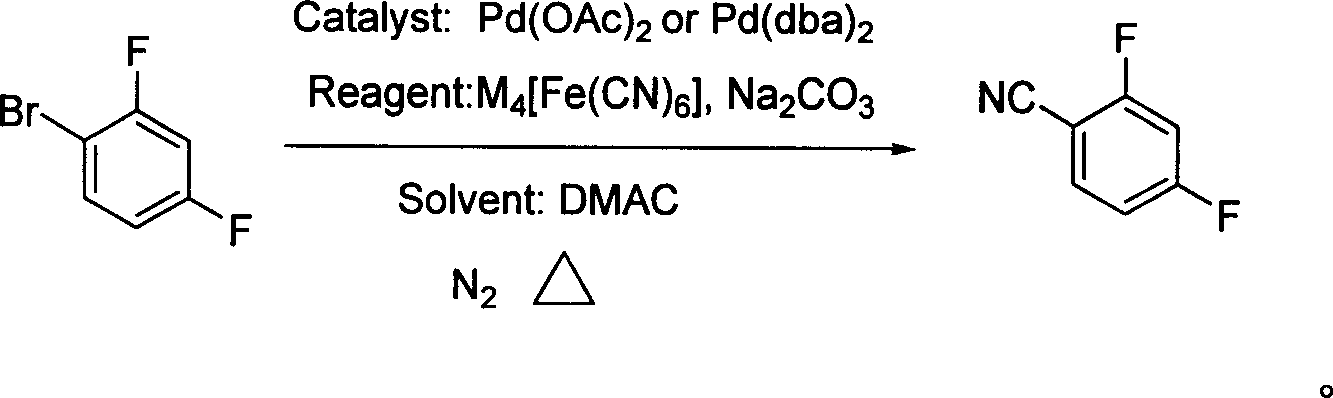Process for preparing 2,4-difluorocyanobenzene
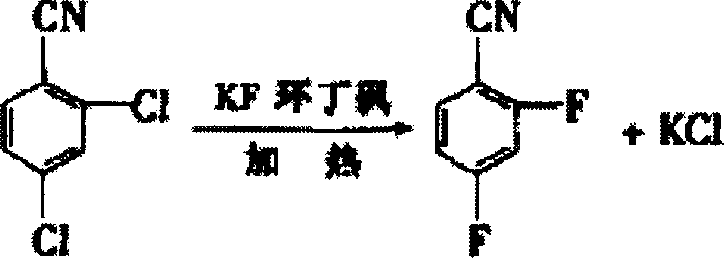
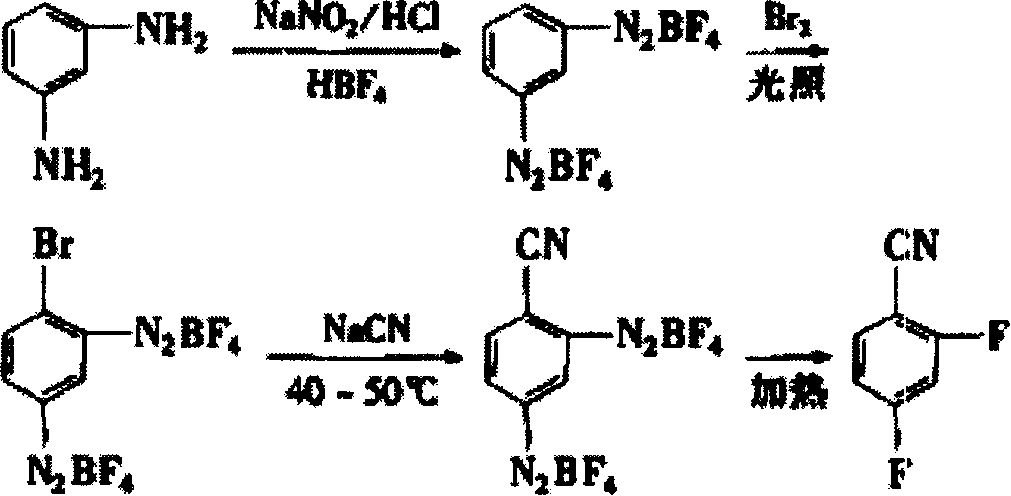
A difluorobenzonitrile and production process technology, applied in two fields, can solve the problems of long reaction process cycle time, complex catalyst preparation, complex post-treatment, etc., and achieve the effects of high yield, low pollution and short reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 1000 ml three-necked flask, under nitrogen protection, 500 ml of N,N-dimethylacetamide solvent, 57.6 g (300 mmol) of 2,4-difluorobromobenzene, 25 g (60 mmol, 0.2 Equivalent) potassium ferrocyanide trihydrate, 680 mg (3 mmol, 0.3 mol%) palladium acetate catalyst, 35 g of sodium carbonate (330 mmol, 1.1 equiv), under nitrogen protection at 100 ° C for 2 hours with stirring, The reaction was completed, followed by filtration, and the filtrate was fractionated under reduced pressure to obtain white crystals of 2,4-difluorobenzonitrile with a yield of 85%, a purity of 98%, a boiling point of 84-86°C (2666.4 Pa), and a melting point of 47-49°C.
Embodiment 2
[0027] In a 1000 ml three-necked flask, under nitrogen protection, 500 ml of N,N-dimethylacetamide solvent, 57.6 g (300 mmol) of 2,4-difluorobromobenzene, 28 g (66 mmol, 0.3 Equivalent) potassium ferrocyanide trihydrate, 340 mg (1.5 mmol, 0.5 mol%) of palladium acetate catalyst, 31.8 g of sodium carbonate (300 mmol, 1.0 equiv.), and the reaction was stirred at 120 ° C for 3 hours under nitrogen protection, The reaction was completed, followed by filtration, and the filtrate was fractionated under reduced pressure to obtain white crystals of 2,4-difluorobenzonitrile with a yield of 90%, a purity of 99%, and a melting point of 48-49°C.
Embodiment 3
[0029] In a 1000 ml three-necked flask, under nitrogen protection, 500 ml of N,N-dimethylacetamide solvent, 57.6 g (300 mmol) of 2,4-difluorobromobenzene, 27 g (66 mmol, 0.22 equiv) sodium ferrocyanide trihydrate, 15 mmol (5 mol %) bis(dibenzylidenepyruvate) palladium catalyst, 31.8 g sodium carbonate (300 mmol, 1.0 equiv), stirred at 120°C under nitrogen protection The reaction was completed for 1 hour, followed by filtration, and the filtrate was fractionated under reduced pressure to obtain 2,4-difluorobenzonitrile as white crystals with a yield of 87%, a purity of 98%, and a melting point of 47-49°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com